当前位置:
X-MOL 学术
›
Prog. Nat. Sci. Mater. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid electrolyte composite Li4P2O7–Li3PO4 for lithium ion battery
Progress in Natural Science: Materials International ( IF 4.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.pnsc.2020.01.020 Evvy Kartini , Valentina Yapriadi , Heri Jodi , Maykel Manawan , Cipta Panghegar , Wahyudianingsih
Progress in Natural Science: Materials International ( IF 4.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.pnsc.2020.01.020 Evvy Kartini , Valentina Yapriadi , Heri Jodi , Maykel Manawan , Cipta Panghegar , Wahyudianingsih
|
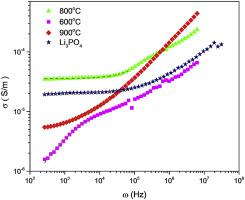
Abstract The researches on solid electrolyte have been significantly increasing due to the safety problem in lithium ion battery. The lithium phosphates are chosen due to environmentally friendly. In the present study Li4P2O7 was synthesized by solid state reaction using NH4H2PO4 and Li2CO3 with the ratio 1:2 at various temperatures of 600 °C, 800 °C and 900 °C. The products were characterized by x-ray diffraction, scanning electron microscopy and impedance spectroscopy. The x-ray diffraction showed that all samples consisted of two phases. It was found that the products consisted of 52.44% Li4P2O7 and 47.56% LiPO3; 93.56% Li4P2O7 and 6.44% Li3PO4; and 46.27% Li4P2O7 and 53.67% Li3PO4 under the synthesizing temperature of 600 °C, 800 °C and 900 °C, respectively. The highest ionic conductivity of 3.85 × 10−5 S/m was achieved for composite Li4P2O7–Li3PO4 with the highest content of 93.56% Li4P2O7. This conductivity is higher compared with single phase of LiPO3, Li3PO4 and Li4P2O7. The increase in ionic conductivity may be due to the mixed anion effects related to the phosphate networks, and it also corresponds to the existence of anorthic phase Li4P2O7 with the space group P −1 (2). The crystal lattice analysis showed that the reactant Li4P2O7 consisted of diphosphate groups P2O7. The lithium tetrahedral LiO4 were linked to P2O7 groups formed a continuous framework containing large voids, available for Li+ ion transport, and thus it exhibited high conductivity. A composite Li4P2O7–Li3PO4 is a promising solid electrolyte for solid state battery.
中文翻译:

锂离子电池用固体电解质复合材料Li4P2O7–Li3PO4
摘要 由于锂离子电池的安全问题,固体电解质的研究显着增加。选择磷酸锂是因为环保。在本研究中,Li4P2O7 是通过固态反应合成的,使用 NH4H2PO4 和 Li2CO3,比例为 1:2,在 600°C、800°C 和 900°C 的不同温度下。通过X射线衍射、扫描电子显微镜和阻抗谱对产物进行表征。X 射线衍射表明所有样品均由两相组成。发现产物由52.44%的Li4P2O7和47.56%的LiPO3组成;93.56% Li4P2O7 和 6.44% Li3PO4;和 46.27% Li4P2O7 和 53.67% Li3PO4 在 600 °C、800 °C 和 900 °C 的合成温度下分别。最高离子电导率3。复合 Li4P2O7–Li3PO4 达到了 85 × 10−5 S/m,Li4P2O7 的最高含量为 93.56%。与 LiPO3、Li3PO4 和 Li4P2O7 的单相相比,这种电导率更高。离子电导率的增加可能是由于与磷酸盐网络相关的混合阴离子效应,也对应于空间群为 P -1 (2) 的正极相 Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。与 LiPO3、Li3PO4 和 Li4P2O7 的单相相比,这种电导率更高。离子电导率的增加可能是由于与磷酸盐网络相关的混合阴离子效应,也对应于空间群为 P -1 (2) 的正极相 Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。与 LiPO3、Li3PO4 和 Li4P2O7 的单相相比,这种电导率更高。离子电导率的增加可能是由于与磷酸盐网络相关的混合阴离子效应,也对应于空间群为 P -1 (2) 的正极相 Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。并且它也对应于空间群为P -1 (2) 的斜相Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。并且它也对应于空间群为P -1 (2) 的斜相Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。
更新日期:2020-04-01
中文翻译:

锂离子电池用固体电解质复合材料Li4P2O7–Li3PO4
摘要 由于锂离子电池的安全问题,固体电解质的研究显着增加。选择磷酸锂是因为环保。在本研究中,Li4P2O7 是通过固态反应合成的,使用 NH4H2PO4 和 Li2CO3,比例为 1:2,在 600°C、800°C 和 900°C 的不同温度下。通过X射线衍射、扫描电子显微镜和阻抗谱对产物进行表征。X 射线衍射表明所有样品均由两相组成。发现产物由52.44%的Li4P2O7和47.56%的LiPO3组成;93.56% Li4P2O7 和 6.44% Li3PO4;和 46.27% Li4P2O7 和 53.67% Li3PO4 在 600 °C、800 °C 和 900 °C 的合成温度下分别。最高离子电导率3。复合 Li4P2O7–Li3PO4 达到了 85 × 10−5 S/m,Li4P2O7 的最高含量为 93.56%。与 LiPO3、Li3PO4 和 Li4P2O7 的单相相比,这种电导率更高。离子电导率的增加可能是由于与磷酸盐网络相关的混合阴离子效应,也对应于空间群为 P -1 (2) 的正极相 Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。与 LiPO3、Li3PO4 和 Li4P2O7 的单相相比,这种电导率更高。离子电导率的增加可能是由于与磷酸盐网络相关的混合阴离子效应,也对应于空间群为 P -1 (2) 的正极相 Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。与 LiPO3、Li3PO4 和 Li4P2O7 的单相相比,这种电导率更高。离子电导率的增加可能是由于与磷酸盐网络相关的混合阴离子效应,也对应于空间群为 P -1 (2) 的正极相 Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。并且它也对应于空间群为P -1 (2) 的斜相Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。并且它也对应于空间群为P -1 (2) 的斜相Li4P2O7 的存在。晶格分析表明反应物Li4P2O7由二磷酸基团P2O7组成。锂四面体 LiO4 与 P2O7 基团连接形成一个包含大空隙的连续框架,可用于 Li+ 离子传输,因此具有高导电性。复合 Li4P2O7–Li3PO4 是一种很有前途的固态电池固体电解质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号