当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming Multidrug Resistance and Biofilms of Pseudomonas aeruginosa with a Single Dual-Function Potentiator of β-Lactams.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-04-06 , DOI: 10.1021/acsinfecdis.9b00486 Anh K Lam 1 , Hannah Panlilio 1 , Jennifer Pusavat 1 , Cassandra L Wouters 1 , Erika L Moen 1 , Charles V Rice 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-04-06 , DOI: 10.1021/acsinfecdis.9b00486 Anh K Lam 1 , Hannah Panlilio 1 , Jennifer Pusavat 1 , Cassandra L Wouters 1 , Erika L Moen 1 , Charles V Rice 1
Affiliation
|
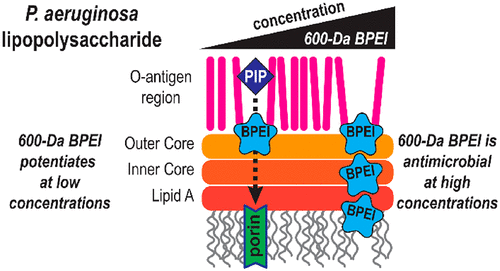
Clinicians prescribe hundreds of millions of β-lactam antibiotics to treat the majority of patients presenting with bacterial infections. Patient outcomes are positive unless resistant bacteria, such as Pseudomonas aeruginosa (P. aeruginosa), are present. P. aeruginosa has both intrinsic and acquired antibiotic resistance, making clinical management of infection a real challenge, particularly when these bacteria are sequestered in biofilms. These problems would be alleviated if, upon the initial presentation of bacterial infection symptoms, clinicians were able to administer an antibiotic that kills both susceptible and otherwise resistant bacteria and eradicates biofilms. As the most common class of antibiotics, β-lactams could be used in a new drug if the leading causes of β-lactam antibiotic resistance, permeation barriers from lipopolysaccharide, efflux pumps, and β-lactamase enzymes, were also defeated. Against P. aeruginosa and their biofilms, the potency of β-lactam antibiotics is restored with 600 Da branched polyethylenimine (600 Da BPEI). Checkerboard assays using microtiter plates demonstrate the potentiation of piperacillin, cefepime, Meropenem, and erythromycin antibiotics. Growth curves demonstrate that only a combination of 600 Da BPEI and piperacillin produces growth inhibition against antibiotic resistant P. aeruginosa. Scanning electron microscopy (SEM) was used to confirm that the combination treatment leads to abnormal P. aeruginosa morphology. Data collected with isothermal titration calorimetry and fluorescence spectroscopy demonstrate a mechanism of action in which potentiation at low concentrations of 600 Da BPEI reduces diffusion barriers from lipopolysaccharides without disrupting the outer membrane itself. Coupled with the ability to overcome a reduction in antibiotic activity created by biofilm exopolymers, targeting anionic sites on lipopolysaccharides and biofilm exopolysaccharides with the same compound provides new opportunities to counter the rise of multidrug-resistant infections.
中文翻译:

使用单一 β-内酰胺双功能增效剂克服铜绿假单胞菌的多药耐药性和生物膜。
临床医生开出数亿种β-内酰胺抗生素来治疗大多数出现细菌感染的患者。除非存在铜绿假单胞菌 (P. aeruginosa) 等耐药细菌,否则患者的治疗结果是积极的。铜绿假单胞菌具有内在和获得性抗生素耐药性,这使得感染的临床管理成为真正的挑战,特别是当这些细菌被隔离在生物膜中时。如果在细菌感染症状最初出现时,临床医生能够使用抗生素杀死敏感细菌和其他耐药细菌并根除生物膜,这些问题将会得到缓解。作为最常见的一类抗生素,如果β-内酰胺抗生素耐药性的主要原因——脂多糖、外排泵和β-内酰胺酶的渗透屏障也被克服,那么β-内酰胺就可以用于新药。针对铜绿假单胞菌及其生物膜,600 Da 支链聚乙烯亚胺 (600 Da BPEI) 可恢复 β-内酰胺抗生素的效力。使用微量滴定板的棋盘测定证明了哌拉西林、头孢吡肟、美罗培南和红霉素抗生素的增强作用。生长曲线表明,只有 600 Da BPEI 和哌拉西林的组合才能对抗生素耐药性铜绿假单胞菌产生生长抑制。使用扫描电子显微镜(SEM)证实联合处理导致铜绿假单胞菌形态异常。通过等温滴定量热法和荧光光谱收集的数据证明了一种作用机制,其中低浓度 600 Da BPEI 的增强作用可减少脂多糖的扩散障碍,而不破坏外膜本身。再加上克服生物膜外聚合物造成的抗生素活性降低的能力,用相同的化合物靶向脂多糖和生物膜外多糖上的阴离子位点,为对抗多重耐药感染的增加提供了新的机会。
更新日期:2020-03-30
中文翻译:

使用单一 β-内酰胺双功能增效剂克服铜绿假单胞菌的多药耐药性和生物膜。
临床医生开出数亿种β-内酰胺抗生素来治疗大多数出现细菌感染的患者。除非存在铜绿假单胞菌 (P. aeruginosa) 等耐药细菌,否则患者的治疗结果是积极的。铜绿假单胞菌具有内在和获得性抗生素耐药性,这使得感染的临床管理成为真正的挑战,特别是当这些细菌被隔离在生物膜中时。如果在细菌感染症状最初出现时,临床医生能够使用抗生素杀死敏感细菌和其他耐药细菌并根除生物膜,这些问题将会得到缓解。作为最常见的一类抗生素,如果β-内酰胺抗生素耐药性的主要原因——脂多糖、外排泵和β-内酰胺酶的渗透屏障也被克服,那么β-内酰胺就可以用于新药。针对铜绿假单胞菌及其生物膜,600 Da 支链聚乙烯亚胺 (600 Da BPEI) 可恢复 β-内酰胺抗生素的效力。使用微量滴定板的棋盘测定证明了哌拉西林、头孢吡肟、美罗培南和红霉素抗生素的增强作用。生长曲线表明,只有 600 Da BPEI 和哌拉西林的组合才能对抗生素耐药性铜绿假单胞菌产生生长抑制。使用扫描电子显微镜(SEM)证实联合处理导致铜绿假单胞菌形态异常。通过等温滴定量热法和荧光光谱收集的数据证明了一种作用机制,其中低浓度 600 Da BPEI 的增强作用可减少脂多糖的扩散障碍,而不破坏外膜本身。再加上克服生物膜外聚合物造成的抗生素活性降低的能力,用相同的化合物靶向脂多糖和生物膜外多糖上的阴离子位点,为对抗多重耐药感染的增加提供了新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号