当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the active isomer of cycloalanopine, a cyclic opine from Lactobacillus rhamnosus LS8, through synthesis and analog production
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-04-06 , DOI: 10.1039/d0md00033g Isaac Antwi 1, 2, 3, 4 , Sorina Chiorean 1, 2, 3, 4 , Marco J. van Belkum 1, 2, 3, 4 , John C. Vederas 1, 2, 3, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-04-06 , DOI: 10.1039/d0md00033g Isaac Antwi 1, 2, 3, 4 , Sorina Chiorean 1, 2, 3, 4 , Marco J. van Belkum 1, 2, 3, 4 , John C. Vederas 1, 2, 3, 4
Affiliation
|
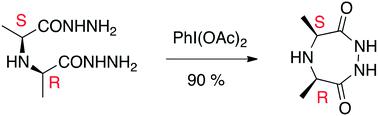
Opines are widely distributed natural products formed by the reductive condensation of amino acids with α-keto acids or carbonyls of carbohydrates. They have important biological roles in bacteria, higher plants, fungi, invertebrates and mammals, including humans. An unusual cyclic opine of undefined stereochemistry, cycloalanopine, was previously isolated from Lactobacillus rhamnosus LS8 and reported to have antimicrobial activity against both Gram-negative and Gram-positive bacteria. In this work, we report a three-step strategy to synthetically access pure isomers of this cyclic compound and analogs thereof. In the key step, acyclic bis-hydrazides can be oxidized with (diacetoxyiodo) benzene to corresponding cyclic N,N′-diacylhydrazides. The three cycloalanopine isomers, along with several analogs, were synthesized and tested against a panel of Gram-positive and Gram-negative bacteria. We identified the active isomer as the meso compound: (4R,6S)-4,6-dimethyl-1,2,5-triazepan-3,7-dione. Additionally, a glycine derivative, (R)-4-methyl-1,2,5-triazepan-3,7-dione, was ascertained to be more potent. This compound was active against both Gram-positive and Gram-negative organisms with the strongest potency against Escherichia coli and Acinetobacter baumannii, an opportunistic pathogen found in hospital-derived infections.
中文翻译:

通过合成和类似物生产来自鼠李糖乳杆菌LS8的环状阿片环果碱的活性异构体
高山是广泛分布的天然产物,是通过氨基酸与α-酮酸或碳水化合物的羰基还原缩合形成的。它们在细菌,高等植物,真菌,无脊椎动物和哺乳动物(包括人类)中具有重要的生物学作用。先前从鼠李糖乳杆菌LS8中分离出了一种不明的立体化学结构的环状阿片环菌素,该化合物对革兰氏阴性菌和革兰氏阳性菌均具有抗菌活性。在这项工作中,我们报告了三步策略来合成访问该环状化合物及其类似物的纯异构体。在关键步骤中,无环双酰肼可被(二乙酰氧基碘)苯氧化为相应的环N,N'-二酰肼。合成了三种环铝环烷异构体以及几种类似物,并针对一组革兰氏阳性和革兰氏阴性细菌进行了测试。我们确定了活性异构体为内消旋化合物:(4 R,6 S)-4,6-二甲基-1,2,5-三氮杂pan-3,7-二酮。另外,确定甘氨酸衍生物(R)-4-甲基-1,2,5-三氮杂潘-3,7-二酮更有效。该化合物对革兰氏阳性和革兰氏阴性生物都具有活性,对大肠杆菌和鲍曼不动杆菌具有最强的效力,鲍曼不动杆菌是在医院感染中发现的机会病原体。
更新日期:2020-04-06
中文翻译:

通过合成和类似物生产来自鼠李糖乳杆菌LS8的环状阿片环果碱的活性异构体
高山是广泛分布的天然产物,是通过氨基酸与α-酮酸或碳水化合物的羰基还原缩合形成的。它们在细菌,高等植物,真菌,无脊椎动物和哺乳动物(包括人类)中具有重要的生物学作用。先前从鼠李糖乳杆菌LS8中分离出了一种不明的立体化学结构的环状阿片环菌素,该化合物对革兰氏阴性菌和革兰氏阳性菌均具有抗菌活性。在这项工作中,我们报告了三步策略来合成访问该环状化合物及其类似物的纯异构体。在关键步骤中,无环双酰肼可被(二乙酰氧基碘)苯氧化为相应的环N,N'-二酰肼。合成了三种环铝环烷异构体以及几种类似物,并针对一组革兰氏阳性和革兰氏阴性细菌进行了测试。我们确定了活性异构体为内消旋化合物:(4 R,6 S)-4,6-二甲基-1,2,5-三氮杂pan-3,7-二酮。另外,确定甘氨酸衍生物(R)-4-甲基-1,2,5-三氮杂潘-3,7-二酮更有效。该化合物对革兰氏阳性和革兰氏阴性生物都具有活性,对大肠杆菌和鲍曼不动杆菌具有最强的效力,鲍曼不动杆菌是在医院感染中发现的机会病原体。









































 京公网安备 11010802027423号
京公网安备 11010802027423号