当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improving the biodegradability of hospital urines polluted with chloramphenicol by the application of electrochemical oxidation.
Science of the Total Environment ( IF 9.8 ) Pub Date : 2020-04-04 , DOI: 10.1016/j.scitotenv.2020.138430 Miguel Herraiz-Carboné 1 , Salvador Cotillas 1 , Engracia Lacasa 1 , Ángela Moratalla 2 , Pablo Cañizares 2 , Manuel A Rodrigo 2 , Cristina Sáez 2
Science of the Total Environment ( IF 9.8 ) Pub Date : 2020-04-04 , DOI: 10.1016/j.scitotenv.2020.138430 Miguel Herraiz-Carboné 1 , Salvador Cotillas 1 , Engracia Lacasa 1 , Ángela Moratalla 2 , Pablo Cañizares 2 , Manuel A Rodrigo 2 , Cristina Sáez 2
Affiliation
|
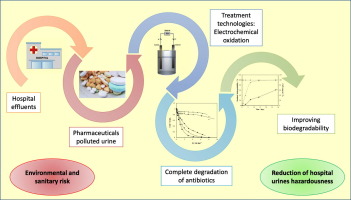
This work focuses on improving the biodegradability of hospital urines polluted with antibiotics by electrochemical advanced oxidation processes (EAOPs). To do this, chloramphenicol (CAP) has been used as a model compound and the influence of anodic material (Boron Doped Diamond (BDD) and Mixed Metal Oxide (MMO)) and current density (1.25-5 mA cm-2) on the toxicity and the biodegradability was evaluated. Results show that a complete CAP removal was attained using BDD anodes, being the process more efficient at the lowest current density tested (1.25 mA cm-2). Conversely, after passing 4 Ah dm-3, only 35% of CAP removal is reached using MMO anodes, regardless of the current density applied. Furthermore, a kinetic study demonstrated that there is a clear competitive oxidation between the target antibiotic and the organic compounds naturally contained in urine, regardless the current density and the anode material used. During the first stages of the electrolysis, acute toxicity is around 1% EC50 but it increases once CAP and its organic intermediates have been degraded. The formation and accumulation of inorganic oxidants may justify the remaining acute toxicity. This also helps to explain the trend observed in the rapid biodegradability assays. Finally, a 60% of standard biodegradability (Zahn-Wellens test) was achieved which suggests that electrochemical oxidation with BDD anodes could be the most appropriate technology to reduce the hazard of hospital urines at the operating conditions tested.
中文翻译:

通过应用电化学氧化提高被氯霉素污染的医院尿液的生物降解性。
这项工作的重点是通过电化学高级氧化工艺(EAOP)改善被抗生素污染的医院尿液的生物降解性。为此,已使用氯霉素(CAP)作为模型化合物,并且阳极材料(掺硼金刚石(BDD)和混合金属氧化物(MMO))和电流密度(1.25-5 mA cm-2)的影响对材料的影响。评估毒性和生物降解性。结果表明,使用BDD阳极可以完全去除CAP,这是在最低电流密度(1.25 mA cm-2)下该过程更为有效的方法。相反,在通过4 Ah dm-3之后,使用MMO阳极仅能达到35%的CAP去除率,而与施加的电流密度无关。此外,动力学研究表明,不管电流密度和使用的阳极材料如何,目标抗生素和尿液中自然含有的有机化合物之间都存在明显的竞争性氧化。在电解的第一阶段,急性毒性约为EC50的1%,但是一旦CAP及其有机中间体被降解,毒性就会增加。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。无论电流密度和使用的阳极材料如何。在电解的第一阶段,急性毒性约为EC50的1%,但是一旦CAP及其有机中间体被降解,毒性就会增加。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。无论电流密度和使用的阳极材料如何。在电解的第一阶段,急性毒性约为EC50的1%,但是一旦CAP及其有机中间体被降解,毒性就会增加。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最后,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以减少在所测试的工作条件下医院尿液的危害。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。
更新日期:2020-04-06
中文翻译:

通过应用电化学氧化提高被氯霉素污染的医院尿液的生物降解性。
这项工作的重点是通过电化学高级氧化工艺(EAOP)改善被抗生素污染的医院尿液的生物降解性。为此,已使用氯霉素(CAP)作为模型化合物,并且阳极材料(掺硼金刚石(BDD)和混合金属氧化物(MMO))和电流密度(1.25-5 mA cm-2)的影响对材料的影响。评估毒性和生物降解性。结果表明,使用BDD阳极可以完全去除CAP,这是在最低电流密度(1.25 mA cm-2)下该过程更为有效的方法。相反,在通过4 Ah dm-3之后,使用MMO阳极仅能达到35%的CAP去除率,而与施加的电流密度无关。此外,动力学研究表明,不管电流密度和使用的阳极材料如何,目标抗生素和尿液中自然含有的有机化合物之间都存在明显的竞争性氧化。在电解的第一阶段,急性毒性约为EC50的1%,但是一旦CAP及其有机中间体被降解,毒性就会增加。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。无论电流密度和使用的阳极材料如何。在电解的第一阶段,急性毒性约为EC50的1%,但是一旦CAP及其有机中间体被降解,毒性就会增加。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。无论电流密度和使用的阳极材料如何。在电解的第一阶段,急性毒性约为EC50的1%,但是一旦CAP及其有机中间体被降解,毒性就会增加。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最后,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以减少在所测试的工作条件下医院尿液的危害。无机氧化剂的形成和积累可以证明剩余的急性毒性。这也有助于解释在快速生物降解测定中观察到的趋势。最终,达到了60%的标准生物降解性(Zahn-Wellens测试),这表明使用BDD阳极进行电化学氧化可能是最合适的技术,以降低在所测试的工作条件下医院尿液的危害。



























 京公网安备 11010802027423号
京公网安备 11010802027423号