当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of oxamic acid using dimensionally stable anodes (DSA) based on a mixture of RuO2 and IrO2 nanoparticles.
Chemosphere ( IF 8.1 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.chemosphere.2020.126674 L Carolina Espinoza 1 , Pamela Sepúlveda 2 , Alejandra García 3 , Denis Martins de Godoi 4 , Ricardo Salazar 1
Chemosphere ( IF 8.1 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.chemosphere.2020.126674 L Carolina Espinoza 1 , Pamela Sepúlveda 2 , Alejandra García 3 , Denis Martins de Godoi 4 , Ricardo Salazar 1
Affiliation
|
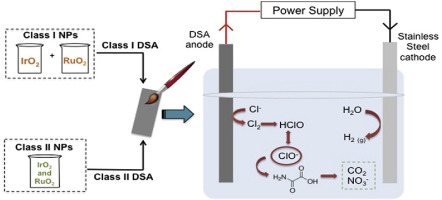
Dimensionally stable anodes (DSA) have been widely used to degrade organic compounds because these surfaces promote the electrogeneration of active chlorine species in the bulk of the solution, as well as in the vicinity of the anode when NaCl is used as supporting electrolyte. In this work, the nanoparticles synthesis of IrO2 and RuO2 was performed to obtain two types of DSA electrodes named Class I and II to degrade oxamic acid. For Class I and II DSA, the nanoparticles used were synthesized separately and in the same reaction medium, respectively. Electrolysis were carried out in an open cylindrical cell without division at 25 °C, DSAs were used as anodes and a stainless-steel electrode as cathode, both elements have a geometric area of 2.8 cm2 immersed in 0.05 mol L-1 of NaCl or Na2SO4 and a current density of 3 mA cm-2 was applied for 6 h. Active chlorine species generated in the absence of oxamic acid in NaCl were also detected and quantified through ion chromatography. In Na2SO4 there was no degradation of the compound, but in NaCl the oxamic acid concentration reaching 85% with Class I DSA. The same tendency is observed in mineralization, in which Class I DSA allowed reaching a CO2 transformation close to 73%. The difference in the results occurs because with Class I DSA, more hypochlorite is generated than with Class II and therefore there is a larger amount of oxidizing species in the solution that enables the degradation and mineralization of oxamic acid.
中文翻译:

使用基于RuO2和IrO2纳米粒子的混合物的尺寸稳定阳极(DSA)降解草酸。
尺寸稳定的阳极(DSA)已被广泛用于降解有机化合物,因为这些表面促进溶液中大部分以及当NaCl用作支持电解质时阳极附近的活性氯物质的电生成。在这项工作中,进行了IrO2和RuO2的纳米粒子合成,从而获得了两种名为I和II类的DSA电极,以降解草酰胺酸。对于I类和II类DSA,所用的纳米颗粒分别在同一反应介质中分别合成。电解在25°C下在不分裂的开放式圆柱形电解槽中进行,DSA用作阳极,不锈钢电极作为阴极,两个元素的几何面积均为2.8 cm2,浸入0.05 mol L-1的NaCl或Na2SO4中并施加3 mA cm-2的电流密度6小时。还通过离子色谱法检测并定量了在NaCl中不存在草酰胺酸时产生的活性氯物质。在Na2SO4中,该化合物没有降解,但在NaCl中,I类DSA的草酰胺酸浓度达到85%。在矿化中观察到了相同的趋势,其中I类DSA允许达到接近73%的CO2转化率。之所以会出现结果差异,是因为与II类相比,I类DSA生成的次氯酸盐更多,因此溶液中存在大量的氧化物质,能够降解草酰胺酸并使其矿化。但在NaCl中,I类DSA的草酰胺酸浓度达到85%。在矿化中观察到了相同的趋势,其中I类DSA允许达到接近73%的CO2转化率。之所以会出现结果差异,是因为与II类相比,I类DSA生成的次氯酸盐更多,因此溶液中存在大量的氧化物质,能够降解草酰胺酸并使其矿化。但在NaCl中,I类DSA的草酰胺酸浓度达到85%。在矿化中观察到了相同的趋势,其中I类DSA允许达到接近73%的CO2转化率。之所以会出现结果差异,是因为与II类相比,I类DSA生成的次氯酸盐更多,因此溶液中存在大量的氧化物质,能够降解草酰胺酸并使其矿化。
更新日期:2020-04-06
中文翻译:

使用基于RuO2和IrO2纳米粒子的混合物的尺寸稳定阳极(DSA)降解草酸。
尺寸稳定的阳极(DSA)已被广泛用于降解有机化合物,因为这些表面促进溶液中大部分以及当NaCl用作支持电解质时阳极附近的活性氯物质的电生成。在这项工作中,进行了IrO2和RuO2的纳米粒子合成,从而获得了两种名为I和II类的DSA电极,以降解草酰胺酸。对于I类和II类DSA,所用的纳米颗粒分别在同一反应介质中分别合成。电解在25°C下在不分裂的开放式圆柱形电解槽中进行,DSA用作阳极,不锈钢电极作为阴极,两个元素的几何面积均为2.8 cm2,浸入0.05 mol L-1的NaCl或Na2SO4中并施加3 mA cm-2的电流密度6小时。还通过离子色谱法检测并定量了在NaCl中不存在草酰胺酸时产生的活性氯物质。在Na2SO4中,该化合物没有降解,但在NaCl中,I类DSA的草酰胺酸浓度达到85%。在矿化中观察到了相同的趋势,其中I类DSA允许达到接近73%的CO2转化率。之所以会出现结果差异,是因为与II类相比,I类DSA生成的次氯酸盐更多,因此溶液中存在大量的氧化物质,能够降解草酰胺酸并使其矿化。但在NaCl中,I类DSA的草酰胺酸浓度达到85%。在矿化中观察到了相同的趋势,其中I类DSA允许达到接近73%的CO2转化率。之所以会出现结果差异,是因为与II类相比,I类DSA生成的次氯酸盐更多,因此溶液中存在大量的氧化物质,能够降解草酰胺酸并使其矿化。但在NaCl中,I类DSA的草酰胺酸浓度达到85%。在矿化中观察到了相同的趋势,其中I类DSA允许达到接近73%的CO2转化率。之所以会出现结果差异,是因为与II类相比,I类DSA生成的次氯酸盐更多,因此溶液中存在大量的氧化物质,能够降解草酰胺酸并使其矿化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号