当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Polymerization Modification of Polymeric Active Esters towards TEMPO Containing Polymers: A Systematic Study
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.eurpolymj.2020.109660 Wenwen Xue , Hatice Mutlu , Patrick Theato
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.eurpolymj.2020.109660 Wenwen Xue , Hatice Mutlu , Patrick Theato
|
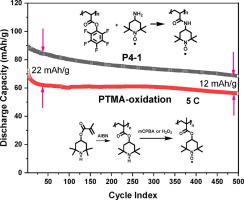
Abstract Organic radical batteries (ORB) are a novel promising class for energy storage, particularly featuring a fast charging capability and extraordinary cycle life. The representative polymer, i.e. poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl methacrylate) (PTMA), is usually synthesized by a post-polymerization oxidation method. As an alternate strategy for developing TEMPO-containing polymers, we focused on the post-polymerization modification of poly(pentafluorophenyl acrylate) and poly(pentafluorophenyl methacrylate) with three different TEMPO nucleophiles by transesterification or amidation reactions. Optimizing the conditions of the post-polymerization functionalization reaction by varying different parameters, such as the type of nucleophile, catalyst and solvent, the feeding ratios of catalysts and nucleophiles, along with reaction time and temperatures, resulted in structurally distinct TEMPO containing polymers with varying backbone composition. Intriguingly, poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl acrylamide) revealed the highest degree functionalization with TEMPO (96.2%) within 3 hrs. under considerably mild conditions, while poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl methylmethacrylamide) exhibited the lowest TEMPO content owing to the steric hindrance from methyl group on both the methacrylate chain and the TEMPO derivative. All other four TEMPO containing polymers had a radical content similar to PTMA (66.6%) synthesized by the post-oxidation methodology. Noteworthy, compared to TEA (trimethylamine), DMAP (4-dimethylaminopyridine) facilitated an efficient ester bond cleavage independent of the polymer precursor, thus, side reactions such as hydrolysis were increased. Though hydrolysis side reaction occurred, the resulting carboxylic acid group was proven to accelerate ion transfer in a certain way during the redox process. Furthermore, due to the higher TEMPO content, poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl acrylamide) exhibited a 12–22 mAh/g higher specific capacity compared to the PTMA-oxidation when running at 5C for 500 cycles.
中文翻译:

聚合活性酯对含 TEMPO 聚合物的聚合后改性:系统研究
摘要 有机自由基电池(ORB)是一种新型的有前途的储能类别,尤其具有快速充电能力和非凡的循环寿命。代表性聚合物,即聚(2,2,6,6-四甲基哌啶氧基-4-甲基丙烯酸酯)(PTMA),通常通过聚合后氧化法合成。作为开发含 TEMPO 聚合物的替代策略,我们专注于通过酯交换或酰胺化反应使用三种不同的 TEMPO 亲核试剂对聚(五氟苯基丙烯酸酯)和聚(甲基丙烯酸五氟苯基酯)进行聚合后改性。通过改变亲核试剂的类型、催化剂和溶剂的类型、催化剂和亲核试剂的进料比等不同的参数来优化聚合后官能化反应的条件,随着反应时间和温度的变化,产生了结构不同的含有 TEMPO 的聚合物,其主链组成不同。有趣的是,聚(2,2,6,6-四甲基哌啶氧基-4-基丙烯酰胺)在 3 小时内显示出最高程度的 TEMPO 官能化(96.2%)。在相当温和的条件下,聚(2,2,6,6-四甲基哌啶氧基-4-基甲基甲基丙烯酰胺)由于甲基丙烯酸酯链和 TEMPO 衍生物上的甲基的空间位阻而表现出最低的 TEMPO 含量。所有其他四种含有 TEMPO 的聚合物的自由基含量与通过后氧化方法合成的 PTMA (66.6%) 相似。值得注意的是,与 TEA(三甲胺)相比,DMAP(4-二甲氨基吡啶)促进了独立于聚合物前体的有效酯键断裂,因此,水解等副反应增加。尽管发生了水解副反应,但证明生成的羧酸基团在氧化还原过程中以某种方式加速了离子转移。此外,由于较高的 TEMPO 含量,当在 5C 下运行 500 次循环时,聚(2,2,6,6-四甲基哌啶氧基-4-基丙烯酰胺)与 PTMA 氧化相比表现出更高的比容量 12-22 mAh/g .
更新日期:2020-05-01
中文翻译:

聚合活性酯对含 TEMPO 聚合物的聚合后改性:系统研究
摘要 有机自由基电池(ORB)是一种新型的有前途的储能类别,尤其具有快速充电能力和非凡的循环寿命。代表性聚合物,即聚(2,2,6,6-四甲基哌啶氧基-4-甲基丙烯酸酯)(PTMA),通常通过聚合后氧化法合成。作为开发含 TEMPO 聚合物的替代策略,我们专注于通过酯交换或酰胺化反应使用三种不同的 TEMPO 亲核试剂对聚(五氟苯基丙烯酸酯)和聚(甲基丙烯酸五氟苯基酯)进行聚合后改性。通过改变亲核试剂的类型、催化剂和溶剂的类型、催化剂和亲核试剂的进料比等不同的参数来优化聚合后官能化反应的条件,随着反应时间和温度的变化,产生了结构不同的含有 TEMPO 的聚合物,其主链组成不同。有趣的是,聚(2,2,6,6-四甲基哌啶氧基-4-基丙烯酰胺)在 3 小时内显示出最高程度的 TEMPO 官能化(96.2%)。在相当温和的条件下,聚(2,2,6,6-四甲基哌啶氧基-4-基甲基甲基丙烯酰胺)由于甲基丙烯酸酯链和 TEMPO 衍生物上的甲基的空间位阻而表现出最低的 TEMPO 含量。所有其他四种含有 TEMPO 的聚合物的自由基含量与通过后氧化方法合成的 PTMA (66.6%) 相似。值得注意的是,与 TEA(三甲胺)相比,DMAP(4-二甲氨基吡啶)促进了独立于聚合物前体的有效酯键断裂,因此,水解等副反应增加。尽管发生了水解副反应,但证明生成的羧酸基团在氧化还原过程中以某种方式加速了离子转移。此外,由于较高的 TEMPO 含量,当在 5C 下运行 500 次循环时,聚(2,2,6,6-四甲基哌啶氧基-4-基丙烯酰胺)与 PTMA 氧化相比表现出更高的比容量 12-22 mAh/g .









































 京公网安备 11010802027423号
京公网安备 11010802027423号