Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.cej.2020.124909 Li Feng , Shengtao Zhang , Yangyang Feng , Xiaolei Ren , Hao Lu , Bochuan Tan , Shijin Chen
|
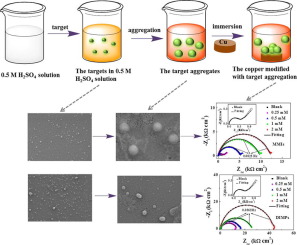
Unilateral and bilateral substitution compounds named 4-((1H-indol-3-yl)methyl)phenol (IMP) and 4-(di(1H-indol-3-yl)methyl)phenol (DIMP) have been synthesized, basing on the preparation of copolymer. The morphology images show that the target molecules can aggregate and organize spontaneously in sulfuric acid solution at room temperature with the formation of spherical or ellipsoidal nano-copolymers. And the effects of structure of synthesized compounds on copper corrosion inhibition are analyzed. The XPS study indicates that the organization of copolymers on the copper surface is mainly forced by the chemical adsorption, that is, the N heteroatom contained lone pair electrons can give electrons to the vacant orbital of copper as a result of forming the adsorbed protection layer. The electrochemical tests suggest that target copolymers show excellent corrosion protection performance for copper in 0.5 M H2SO4 solution. The corrosion inhibition efficiency of DIMP aggregates (DIMPs, 99.3%) is better than the IMP aggregates (IMPs, 97.5%) at the concentration of 2 mM, which is attributed to the effects of the stronger intermolecular hydrogen bonding and π-π intermolecular forces of bilateral donated groups of DIMPs than that of IMPs. In addition, the improvement of hydrophobicity of target-modification is also in favor of inhibition performance. Theoretical calculations further reveal the active sites of inhibitor molecules and their adsorption configurations on the copper surface.
中文翻译:

新型合成化合物的自聚集纳米级共聚物,可有效保护硫酸溶液中的铜腐蚀
在此基础上,合成了名为4-(((1H-吲哚-3-基)甲基)苯酚(IMP)和4-(二(1H-吲哚-3-基)甲基)苯酚(DIMP)的单侧和双边取代化合物。共聚物的制备。形态图像表明,在室温下,目标分子可以在硫酸溶液中自发聚集和组织,形成球形或椭圆形纳米共聚物。并分析了合成化合物的结构对缓蚀铜的影响。XPS研究表明,铜表面上共聚物的组织主要是由化学吸附作用所致,也就是说,由于形成了吸附保护层,N杂原子所含的孤对电子可以将电子赋予铜的空轨道。2 SO 4解决方案。在浓度为2 mM时,DIMP聚集体(DIMPs,99.3%)的腐蚀抑制性能优于IMP聚集体(IMPs,97.5%),这归因于更强的分子间氢键和π-π分子间力双边捐赠的DIMP组比IMP的捐赠组多。另外,靶修饰的疏水性的改善也有利于抑制性能。理论计算进一步揭示了抑制剂分子的活性位及其在铜表面的吸附构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号