当前位置:
X-MOL 学术
›
J. Mol. Cell. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human Purkinje in silico model enables mechanistic investigations into automaticity and pro-arrhythmic abnormalities.
Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.yjmcc.2020.04.001 Cristian Trovato 1 , Elisa Passini 1 , Norbert Nagy 2 , András Varró 2 , Najah Abi-Gerges 3 , Stefano Severi 4 , Blanca Rodriguez 1
Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.yjmcc.2020.04.001 Cristian Trovato 1 , Elisa Passini 1 , Norbert Nagy 2 , András Varró 2 , Najah Abi-Gerges 3 , Stefano Severi 4 , Blanca Rodriguez 1
Affiliation
|
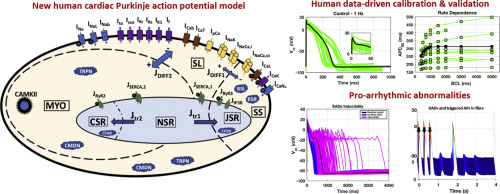
Cardiac Purkinje cells (PCs) are implicated in lethal arrhythmias caused by cardiac diseases, mutations, and drug action. However, the pro-arrhythmic mechanisms in PCs are not entirely understood, particularly in humans, as most investigations are conducted in animals. The aims of this study are to present a novel human PCs electrophysiology biophysically-detailed computational model, and to disentangle ionic mechanisms of human Purkinje-related electrophysiology, pacemaker activity and arrhythmogenicity. The new Trovato2020 model incorporates detailed Purkinje-specific ionic currents and Ca2+ handling, and was developed, calibrated and validated using human experimental data acquired at multiple frequencies, both in control conditions and following drug application. Multiscale investigations were performed in a Purkinje cell, in fibre and using an experimentally-calibrated population of PCs to evaluate biological variability. Simulations demonstrate the human Purkinje Trovato2020 model is the first one to yield: (i) all key AP features consistent with human Purkinje recordings; (ii) Automaticity with funny current up-regulation (iii) EADs at slow pacing and with 85% hERG block; (iv) DADs following fast pacing; (v) conduction velocity of 160 cm/s in a Purkinje fibre, as reported in human. The human in silico PCs population highlights that: (1) EADs are caused by ICaL reactivation in PCs with large inward currents; (2) DADs and triggered APs occur in PCs experiencing Ca2+ accumulation, at fast pacing, caused by large L-type calcium current and small Na+/Ca2+ exchanger. The novel human Purkinje model unlocks further investigations into the role of cardiac Purkinje in ventricular arrhythmias through computer modeling and multiscale simulations.
中文翻译:

人类浦肯野计算机模型可以对自动性和促心律失常异常进行机械研究。
心脏浦肯野细胞 (PC) 与心脏病、突变和药物作用引起的致命性心律失常有关。然而,PC 的促心律失常机制尚不完全清楚,特别是在人类中,因为大多数研究都是在动物中进行的。本研究的目的是提出一种新型的人类 PC 电生理学生物物理详细计算模型,并阐明人类浦肯野相关电生理学、起搏器活动和心律失常的离子机制。新的 Trovato2020 模型结合了详细的浦肯野特定离子电流和 Ca2+ 处理,并使用在控制条件和药物应用后的多个频率下获取的人体实验数据进行开发、校准和验证。在浦肯野细胞、纤维中进行多尺度研究,并使用经过实验校准的 PC 群体来评估生物变异性。模拟表明,人类 Purkinje Trovato2020 模型是第一个能够产生以下结果的模型:(i) 所有关键 AP 特征与人类 Purkinje 记录一致; (ii) 具有有趣的电流上调的自动性 (iii) 慢速 EAD 和 85% hERG 阻滞; (iv) 快节奏的 DAD; (v) 根据人体报道,浦肯野纤维的传导速度为 160 厘米/秒。人类计算机 PC 群体强调:(1)EAD 是由具有大内向电流的 PC 中 ICaL 重新激活引起的; (2) DAD 和触发的 AP 发生在经历 Ca2+ 积累的 PC 中,速度快,由大的 L 型钙电流和小的 Na+/Ca2+ 交换器引起。新型人类浦肯野模型通过计算机建模和多尺度模拟,进一步研究了心脏浦肯野在室性心律失常中的作用。
更新日期:2020-04-06
中文翻译:

人类浦肯野计算机模型可以对自动性和促心律失常异常进行机械研究。
心脏浦肯野细胞 (PC) 与心脏病、突变和药物作用引起的致命性心律失常有关。然而,PC 的促心律失常机制尚不完全清楚,特别是在人类中,因为大多数研究都是在动物中进行的。本研究的目的是提出一种新型的人类 PC 电生理学生物物理详细计算模型,并阐明人类浦肯野相关电生理学、起搏器活动和心律失常的离子机制。新的 Trovato2020 模型结合了详细的浦肯野特定离子电流和 Ca2+ 处理,并使用在控制条件和药物应用后的多个频率下获取的人体实验数据进行开发、校准和验证。在浦肯野细胞、纤维中进行多尺度研究,并使用经过实验校准的 PC 群体来评估生物变异性。模拟表明,人类 Purkinje Trovato2020 模型是第一个能够产生以下结果的模型:(i) 所有关键 AP 特征与人类 Purkinje 记录一致; (ii) 具有有趣的电流上调的自动性 (iii) 慢速 EAD 和 85% hERG 阻滞; (iv) 快节奏的 DAD; (v) 根据人体报道,浦肯野纤维的传导速度为 160 厘米/秒。人类计算机 PC 群体强调:(1)EAD 是由具有大内向电流的 PC 中 ICaL 重新激活引起的; (2) DAD 和触发的 AP 发生在经历 Ca2+ 积累的 PC 中,速度快,由大的 L 型钙电流和小的 Na+/Ca2+ 交换器引起。新型人类浦肯野模型通过计算机建模和多尺度模拟,进一步研究了心脏浦肯野在室性心律失常中的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号