当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.030 Weiwei Huang 1 , Congyan Shu 2 , Liangqun Hua 3 , Yilin Zhao 4 , Hanghang Xie 1 , Jialong Qi 1 , Fulan Gao 1 , Ruiyu Gao 1 , Yongjun Chen 1 , Qishu Zhang 1 , Weiran Li 1 , Mingcui Yuan 1 , Chao Ye 1 , Yanbing Ma 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.030 Weiwei Huang 1 , Congyan Shu 2 , Liangqun Hua 3 , Yilin Zhao 4 , Hanghang Xie 1 , Jialong Qi 1 , Fulan Gao 1 , Ruiyu Gao 1 , Yongjun Chen 1 , Qishu Zhang 1 , Weiran Li 1 , Mingcui Yuan 1 , Chao Ye 1 , Yanbing Ma 1
Affiliation
|
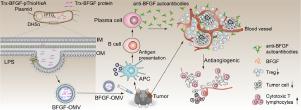
Using monoclonal antibodies to block tumor angiogenesis has yielded effective antitumor effects. However, this treatment method has long cycles and is very expensive; therefore, its long-term and extensive application is limited. In this study, we developed a nanovaccine using bacterial biomembranes as carriers for antitumor therapy. The whole basic fibroblast growth factor (BFGF) molecule (154 amino acids (aa)) was loaded onto bacterial outer membrane vesicles (OMVs) using gene recombination technology. The strong adjuvant effect of OMVs was used to induce the host to produce anti-BFGF autoantibodies. We proved that persistent anti-BFGF autoantibodies can be induced in mice after only 3 immunizations to antagonize BFGF functions. The effects included multiple tumor suppression functions, including inhibition of tumor angiogenesis, induction of tumor cell apoptosis, reversal of tumor immune barriers, and promotion of tumor-specific cytotoxic T lymphocytes (CTLs), eventually causing tumor regression. We confirmed that bacterial biomembranes can be used as a vaccine delivery system to induce the production of antibodies against autoantigens, which may be used for tumor therapy. This study expands the application fields of bacterial biomembrane systems and provides insight for tumor immunotherapy other than monoclonal antibody technology. STATEMENT OF SIGNIFICANCE: In this study, we proved that bacteria-released outer membrane vesicles (OMVs) modified via genetic engineering can be used as a vaccine carrier to break autoimmune tolerance and induce the body to produce autoantibodies to antagonize pathological molecules and block pathological signaling pathways for tumor therapy. OMVs naturally released by bacteria were used to successfully load the full-length BFGF protein (154 aa). We proved that persistent anti-BFGF autoantibodies can be induced in tumor-bearing mice after only 3 immunizations to effectively inhibit tumors. Furthermore, the production of these antibodies successfully inhibited tumor angiogenesis, promoted tumor cell apoptosis, reversed the tumor immunosuppressive microenvironment, increased the cytotoxic T lymphocyte (CTL) reaction, and eventually inhibited tumor growth.
中文翻译:

修饰的细菌外膜囊泡诱导自身抗体用于肿瘤治疗。
使用单克隆抗体阻断肿瘤血管生成已产生有效的抗肿瘤作用。但是,这种处理方法周期长且非常昂贵。因此,其长期广泛的应用受到限制。在这项研究中,我们开发了一种纳米细菌疫苗,使用细菌生物膜作为载体进行抗肿瘤治疗。使用基因重组技术将整个基本成纤维细胞生长因子(BFGF)分子(154个氨基酸(aa))加载到细菌外膜囊泡(OMV)上。OMV的强佐剂作用被用来诱导宿主产生抗BFGF自身抗体。我们证明了仅3次免疫拮抗BFGF功能的免疫后就可以在小鼠中诱导持久性抗BFGF自身抗体。这些作用包括多种肿瘤抑制功能,包括抑制肿瘤血管生成,诱导肿瘤细胞凋亡,逆转肿瘤免疫屏障以及促进肿瘤特异性细胞毒性T淋巴细胞(CTL),最终导致肿瘤消退。我们证实细菌生物膜可用作疫苗递送系统,以诱导产生针对自身抗原的抗体,该抗体可用于肿瘤治疗。这项研究扩展了细菌生物膜系统的应用领域,并为除单克隆抗体技术以外的肿瘤免疫疗法提供了见识。重大意义声明:在这项研究中,我们证明了通过基因工程修饰的细菌释放的外膜囊泡(OMV)可以用作疫苗载体来破坏自身免疫耐受,并诱导人体产生自身抗体来拮抗病理分子并阻断病理信号肿瘤治疗的途径。细菌天然释放的OMV用于成功加载全长BFGF蛋白(154aa)。我们证明,仅需3次免疫即可有效抑制肿瘤,从而在荷瘤小鼠中诱导出持久性抗BFGF自身抗体。此外,这些抗体的产生成功抑制了肿瘤血管生成,促进了肿瘤细胞凋亡,逆转了肿瘤免疫抑制微环境,增加了细胞毒性T淋巴细胞(CTL)反应,并最终抑制了肿瘤的生长。
更新日期:2020-04-03
中文翻译:

修饰的细菌外膜囊泡诱导自身抗体用于肿瘤治疗。
使用单克隆抗体阻断肿瘤血管生成已产生有效的抗肿瘤作用。但是,这种处理方法周期长且非常昂贵。因此,其长期广泛的应用受到限制。在这项研究中,我们开发了一种纳米细菌疫苗,使用细菌生物膜作为载体进行抗肿瘤治疗。使用基因重组技术将整个基本成纤维细胞生长因子(BFGF)分子(154个氨基酸(aa))加载到细菌外膜囊泡(OMV)上。OMV的强佐剂作用被用来诱导宿主产生抗BFGF自身抗体。我们证明了仅3次免疫拮抗BFGF功能的免疫后就可以在小鼠中诱导持久性抗BFGF自身抗体。这些作用包括多种肿瘤抑制功能,包括抑制肿瘤血管生成,诱导肿瘤细胞凋亡,逆转肿瘤免疫屏障以及促进肿瘤特异性细胞毒性T淋巴细胞(CTL),最终导致肿瘤消退。我们证实细菌生物膜可用作疫苗递送系统,以诱导产生针对自身抗原的抗体,该抗体可用于肿瘤治疗。这项研究扩展了细菌生物膜系统的应用领域,并为除单克隆抗体技术以外的肿瘤免疫疗法提供了见识。重大意义声明:在这项研究中,我们证明了通过基因工程修饰的细菌释放的外膜囊泡(OMV)可以用作疫苗载体来破坏自身免疫耐受,并诱导人体产生自身抗体来拮抗病理分子并阻断病理信号肿瘤治疗的途径。细菌天然释放的OMV用于成功加载全长BFGF蛋白(154aa)。我们证明,仅需3次免疫即可有效抑制肿瘤,从而在荷瘤小鼠中诱导出持久性抗BFGF自身抗体。此外,这些抗体的产生成功抑制了肿瘤血管生成,促进了肿瘤细胞凋亡,逆转了肿瘤免疫抑制微环境,增加了细胞毒性T淋巴细胞(CTL)反应,并最终抑制了肿瘤的生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号