当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid bone repair with the recruitment of CD206+M2-like macrophages using non-viral scaffold-mediated miR-133a inhibition of host cells.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.042 Irene Mencía Castaño 1 , Rosanne M Raftery 2 , Gang Chen 3 , Brenton Cavanagh 4 , Brian Quinn 5 , Garry P Duffy 6 , Fergal J O'Brien 1 , Caroline M Curtin 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.042 Irene Mencía Castaño 1 , Rosanne M Raftery 2 , Gang Chen 3 , Brenton Cavanagh 4 , Brian Quinn 5 , Garry P Duffy 6 , Fergal J O'Brien 1 , Caroline M Curtin 1
Affiliation
|
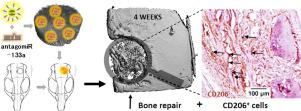
microRNAs offer vast therapeutic potential for multiple disciplines. From a bone perspective, inhibition of miR-133a may offer potential to enhance Runx2 activity and increase bone repair. This study aims to assess the therapeutic capability of antagomiR-133a delivery from collagen-nanohydroxyapatite (coll-nHA) scaffolds following cell-free implantation in rat calvarial defects (7 mm diameter). This is, to the best of our knowledge, the first report of successful in vivo antagomiR uptake in host cells of fully immunocompetent animals without distribution to other off-target tissues. Our results demonstrate the localized release of antagomiR-133a to the implant site at 1 week post-implantation with increased calcium deposits already evident in the antagomiR-133a loaded scaffolds at this early timepoint. This was followed by an approximate 2-fold increase in bone volume versus antagomiR-free scaffolds and a significant 10-fold increase over the empty defect controls, after just 4 weeks. An increase in host CD206+ cells suggests an accelerated pro-remodeling response by M2-like macrophages accompanying bone repair with this treatment. Overall, this non-viral scaffold-mediated antagomiR-133a delivery platform demonstrates capability to accelerate bone repair in vivo - without the addition of exogenous cells - and underlines the role of M2 macrophage-like cells in directing accelerated bone repair. Expanding the repertoire of this platform to deliver alternative miRNAs offers exciting possibilities for a variety of therapeutic indications. STATEMENT OF SIGNIFICANCE: microRNAs, small non-coding RNA molecules involved in gene regulation, may have potential as a new class of bone healing therapeutics as they can enhance the regenerative capacity of bone-forming cells. We developed a collagen-nanohydroxyapatite-microRNA scaffold system to investigate whether miR133a inhibition can enhance osteogenesis in rat MSCs and ultimately accelerate endogenous bone repair by host cells in vivo without pre-seeding cells prior to implantation. Overall, this off-the-shelf, non-viral scaffold-mediated antagomiR-133a delivery platform demonstrates capability to accelerate bone repair in vivo - without the requirement of exogenous cells - and highlights the role of CD206+M2 macrophage-like cells in guiding accelerated bone repair. Translating the repertoire of this platform to deliver alternative miRNAs offers exciting possibilities for a vast myriad of therapeutic indications.
中文翻译:

使用非病毒支架介导的miR-133a抑制宿主细胞募集CD206 + M2类巨噬细胞,快速修复骨骼。
microRNA为多种学科提供了巨大的治疗潜力。从骨骼的角度看,抑制miR-133a可能会增强Runx2活性并增加骨骼修复能力。这项研究旨在评估无细胞植入大鼠颅盖缺损(直径7 mm)后从胶原蛋白-纳米羟基磷灰石(coll-nHA)支架递送antagomiR-133a的治疗能力。据我们所知,这是第一个成功报道完全免疫能力强的动物的宿主细胞体内antagomiR摄取成功且没有分布到其他脱靶组织的报道。我们的结果表明,在植入后1周,antagomiR-133a局部释放到植入位点,并且在此早期时间点,已经在负载antagomiR-133a的支架中明显地增加了钙沉积。与之相比,仅用了4周的时间,与无antagomiR的支架相比,其骨体积就增加了约2倍,而与空缺损对照组相比,骨量则显着增加了10倍。宿主CD206 +细胞的增加表明,伴随这种治疗的骨修复,M2类巨噬细胞的促重塑反应加快。总体而言,该非病毒支架介导的antagomiR-133a递送平台展示了在不增加外源细胞的情况下,在体内加速骨修复的能力,并强调了M2巨噬细胞样细胞在指导加速的骨修复中的作用。扩展该平台的范围以提供替代性miRNA,可为多种治疗适应症提供令人兴奋的可能性。重要性声明:microRNA,参与基因调控的小型非编码RNA分子,由于它们可以增强骨形成细胞的再生能力,因此可能作为一类新型的骨愈合疗法具有潜力。我们开发了一种胶原蛋白-纳米羟基磷灰石-microRNA支架系统,以研究miR133a抑制作用是否能增强大鼠MSC的成骨性,并最终在不植入种子前预先植入细胞的情况下,通过宿主细胞体内促进内源性骨修复。总体而言,这种现成的,非病毒支架介导的antagomiR-133a递送平台具有加速体内骨骼修复的能力-无需外源细胞-并突出了CD206 + M2巨噬细胞样细胞在指导中的作用加速骨骼修复。转换该平台的库以提供替代miRNA,可为多种治疗适应症提供令人兴奋的可能性。
更新日期:2020-04-03
中文翻译:

使用非病毒支架介导的miR-133a抑制宿主细胞募集CD206 + M2类巨噬细胞,快速修复骨骼。
microRNA为多种学科提供了巨大的治疗潜力。从骨骼的角度看,抑制miR-133a可能会增强Runx2活性并增加骨骼修复能力。这项研究旨在评估无细胞植入大鼠颅盖缺损(直径7 mm)后从胶原蛋白-纳米羟基磷灰石(coll-nHA)支架递送antagomiR-133a的治疗能力。据我们所知,这是第一个成功报道完全免疫能力强的动物的宿主细胞体内antagomiR摄取成功且没有分布到其他脱靶组织的报道。我们的结果表明,在植入后1周,antagomiR-133a局部释放到植入位点,并且在此早期时间点,已经在负载antagomiR-133a的支架中明显地增加了钙沉积。与之相比,仅用了4周的时间,与无antagomiR的支架相比,其骨体积就增加了约2倍,而与空缺损对照组相比,骨量则显着增加了10倍。宿主CD206 +细胞的增加表明,伴随这种治疗的骨修复,M2类巨噬细胞的促重塑反应加快。总体而言,该非病毒支架介导的antagomiR-133a递送平台展示了在不增加外源细胞的情况下,在体内加速骨修复的能力,并强调了M2巨噬细胞样细胞在指导加速的骨修复中的作用。扩展该平台的范围以提供替代性miRNA,可为多种治疗适应症提供令人兴奋的可能性。重要性声明:microRNA,参与基因调控的小型非编码RNA分子,由于它们可以增强骨形成细胞的再生能力,因此可能作为一类新型的骨愈合疗法具有潜力。我们开发了一种胶原蛋白-纳米羟基磷灰石-microRNA支架系统,以研究miR133a抑制作用是否能增强大鼠MSC的成骨性,并最终在不植入种子前预先植入细胞的情况下,通过宿主细胞体内促进内源性骨修复。总体而言,这种现成的,非病毒支架介导的antagomiR-133a递送平台具有加速体内骨骼修复的能力-无需外源细胞-并突出了CD206 + M2巨噬细胞样细胞在指导中的作用加速骨骼修复。转换该平台的库以提供替代miRNA,可为多种治疗适应症提供令人兴奋的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号