当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Co-delivery of NS1 and BMP2 mRNAs to murine pluripotent stem cells leads to enhanced BMP-2 expression and osteogenic differentiation.
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.045 Pinpin Wang 1 , Delphine Logeart-Avramoglou 2 , Hervé Petite 2 , Cristine Goncalves 1 , Patrick Midoux 1 , Federico Perche 1 , Chantal Pichon 3
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.045 Pinpin Wang 1 , Delphine Logeart-Avramoglou 2 , Hervé Petite 2 , Cristine Goncalves 1 , Patrick Midoux 1 , Federico Perche 1 , Chantal Pichon 3
Affiliation
|
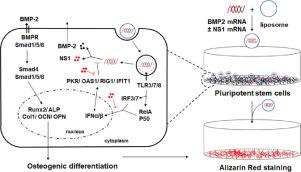
Application of messenger RNA (mRNA) for bone regeneration is a promising alternative to DNA, recombinant proteins and peptides. However, exogenous in vitro transcribed mRNA (IVT mRNA) triggers innate immune response resulting in mRNA degradation and translation inhibition. Inspired by the ability of viral immune evasion proteins to inhibit host cell responses against viral RNA, we applied non-structural protein-1 (NS1) from Influenza A virus (A/Texas/36/1991) as an IVT mRNA enhancer. We evidenced a dose-dependent blocking of RNA sensors by NS1 expression. The co-delivery of NS1 mRNA with mRNA of reporter genes significantly increased the translation efficiency. Interestingly, unlike the use of nucleosides modification, NS1-mediated mRNA translation enhancement does not dependent to cell type. Dual delivery of NS1 mRNA and BMP-2 mRNA to murine pluripotent stem cells (C3H10T1/2), promoted osteogenic differentiation evidenced by enhanced expression of osteoblastic markers (e.g. alkaline phosphatase, type I collagen, osteopontin, and osteocalcin), and extracellular mineralization. Overall, these results support the adjuvant potentiality of NS1 for mRNA-based regenerative therapies. STATEMENT OF SIGNIFICANCE: mRNA therapy has the potential to improve the efficiency of nucleic acid based regenerative medicine. Up to now, the incorporation of expensive modified nucleotides is a common way to avoid IVT mRNA-induced detrimental immunogenicity. We here introduce co-delivery of Influenza virus immune evasion protein-NS1 coding mRNA as a strategy to suppress RNA sensors for maximizing IVT mRNA expression. An increased osteogenic commitment of pluripotent stem cells was observed after BMP2 mRNA and NS1 mRNA delivery. This study revealed how applying non-modified mRNA with NS1 could be a promising alternative as a therapeutic in bone regeneration.
中文翻译:

NS1和BMP2 mRNA向鼠多能干细胞的共同传递导致增强的BMP-2表达和成骨分化。
信使RNA(mRNA)在骨骼再生中的应用是DNA,重组蛋白和肽的有前途的替代方法。但是,外源体外转录的mRNA(IVT mRNA)触发先天免疫反应,导致mRNA降解和翻译抑制。受病毒免疫逃逸蛋白抑制宿主细胞对病毒RNA应答的能力启发,我们应用了甲型流感病毒(A / Texas / 36/1991)的非结构蛋白1(NS1)作为IVT mRNA增强子。我们通过NS1表达证明了RNA传感器的剂量依赖性阻断。NS1 mRNA与报道基因mRNA的共同传递显着提高了翻译效率。有趣的是,与使用核苷修饰不同,NS1介导的mRNA翻译增强不依赖于细胞类型。将NS1 mRNA和BMP-2 mRNA双重递送至鼠多能干细胞(C3H10T1 / 2),可通过成骨细胞标志物(例如碱性磷酸酶,I型胶原,骨桥蛋白和骨钙蛋白)表达增强和细胞外矿化来促进成骨分化。总体而言,这些结果支持NS1在基于mRNA的再生疗法中的辅助潜力。意义声明:mRNA治疗有可能提高基于核酸的再生医学的效率。迄今为止,昂贵的修饰核苷酸的掺入是避免IVT mRNA诱导的有害免疫原性的常用方法。我们在这里介绍流感病毒免疫逃逸蛋白-NS1编码mRNA的共同交付,作为抑制RNA传感器以最大化IVT mRNA表达的策略。BMP2 mRNA和NS1 mRNA交付后,观察到多能干细胞的成骨作用增加。这项研究揭示了如何将未修饰的mRNA与NS1结合使用可以作为一种有望替代骨再生的疗法。
更新日期:2020-04-03
中文翻译:

NS1和BMP2 mRNA向鼠多能干细胞的共同传递导致增强的BMP-2表达和成骨分化。
信使RNA(mRNA)在骨骼再生中的应用是DNA,重组蛋白和肽的有前途的替代方法。但是,外源体外转录的mRNA(IVT mRNA)触发先天免疫反应,导致mRNA降解和翻译抑制。受病毒免疫逃逸蛋白抑制宿主细胞对病毒RNA应答的能力启发,我们应用了甲型流感病毒(A / Texas / 36/1991)的非结构蛋白1(NS1)作为IVT mRNA增强子。我们通过NS1表达证明了RNA传感器的剂量依赖性阻断。NS1 mRNA与报道基因mRNA的共同传递显着提高了翻译效率。有趣的是,与使用核苷修饰不同,NS1介导的mRNA翻译增强不依赖于细胞类型。将NS1 mRNA和BMP-2 mRNA双重递送至鼠多能干细胞(C3H10T1 / 2),可通过成骨细胞标志物(例如碱性磷酸酶,I型胶原,骨桥蛋白和骨钙蛋白)表达增强和细胞外矿化来促进成骨分化。总体而言,这些结果支持NS1在基于mRNA的再生疗法中的辅助潜力。意义声明:mRNA治疗有可能提高基于核酸的再生医学的效率。迄今为止,昂贵的修饰核苷酸的掺入是避免IVT mRNA诱导的有害免疫原性的常用方法。我们在这里介绍流感病毒免疫逃逸蛋白-NS1编码mRNA的共同交付,作为抑制RNA传感器以最大化IVT mRNA表达的策略。BMP2 mRNA和NS1 mRNA交付后,观察到多能干细胞的成骨作用增加。这项研究揭示了如何将未修饰的mRNA与NS1结合使用可以作为一种有望替代骨再生的疗法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号