当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses, in vitro α-amylase and α-glucosidase dual inhibitory activities of 4-amino-1,2,4-triazole derivatives their molecular docking and kinetic studies.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-04-04 , DOI: 10.1016/j.bmc.2020.115467 Emmanuel Oloruntoba Yeye 1 , Kanwal 2 , Khalid Mohammed Khan 3 , Sridevi Chigurupati 4 , Abdul Wadood 5 , Ashfaq Ur Rehman 5 , Shahnaz Perveen 6 , Mari Kannan Maharajan 7 , Shahbaz Shamim 2 , Shehryar Hameed 2 , Sherifat A Aboaba 8 , Muhammad Taha 9
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-04-04 , DOI: 10.1016/j.bmc.2020.115467 Emmanuel Oloruntoba Yeye 1 , Kanwal 2 , Khalid Mohammed Khan 3 , Sridevi Chigurupati 4 , Abdul Wadood 5 , Ashfaq Ur Rehman 5 , Shahnaz Perveen 6 , Mari Kannan Maharajan 7 , Shahbaz Shamim 2 , Shehryar Hameed 2 , Sherifat A Aboaba 8 , Muhammad Taha 9
Affiliation
|
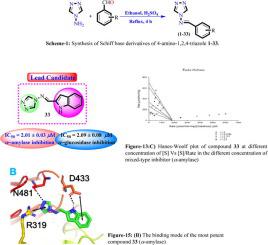
Thirty-three 4-amino-1,2,4-triazole derivatives 1-33 were synthesized by reacting 4-amino-1,2,4-triazole with a variety of benzaldehydes. The synthetic molecules were characterized via1H NMR and EI-MS spectroscopic techniques and evaluated for their anti-hyperglycemic potential. Compounds 1-33 exhibited good to moderate in vitro α-amylase and α-glucosidase inhibitory activities in the range of IC50 values 2.01 ± 0.03-6.44 ± 0.16 and 2.09 ± 0.08-6.54 ± 0.10 µM as compared to the standard acarbose (IC50 = 1.92 ± 0.17 µM) and (IC50 = 1.99 ± 0.07 µM), respectively. The limited structure-activity relationship suggested that different substitutions on aryl part of the synthetic compounds are responsible for variable activity. Kinetic study predicted that compounds 1-33 followed mixed and non-competitive type of inhibitions against α-amylase and α-glucosidase enzymes, respectively. In silico studies revealed that both triazole and aryl ring along with different substitutions were playing an important role in the binding interactions of inhibitors within the enzyme pocket. The synthetic molecules were found to have dual inhibitory potential against both enzymes thus they may serve as lead candidates for the drug development and research in the future studies.
中文翻译:

合成,4-氨基-1,2,4-三唑衍生物的体外α-淀粉酶和α-葡萄糖苷酶双重抑制活性及其分子对接和动力学研究。
通过使4-氨基-1,2,4-三唑与多种苯甲醛反应合成了33种4-氨基-1,2,4-三唑衍生物1-33。合成分子通过1 H NMR和EI-MS光谱技术进行表征,并评估其抗高血糖的潜力。与标准阿卡波糖相比,化合物1-33在IC50值范围为2.01±0.03-6.44±0.16和2.09±0.08-6.54±0.10 µM的范围内,具有良好至中等的体外α-淀粉酶和α-葡萄糖苷酶抑制活性。 1.92±0.17 µM)和(IC50 = 1.99±0.07 µM)。有限的结构活性关系表明,合成化合物芳基部分的不同取代是造成可变活性的原因。动力学研究预测,化合物1-33分别受到混合和非竞争性抑制α-淀粉酶和α-葡萄糖苷酶的作用。在计算机研究中,三唑和芳基环以及不同的取代在酶口袋内抑制剂的结合相互作用中起着重要作用。已发现合成分子对两种酶均具有双重抑制潜能,因此它们可作为药物开发和未来研究的主要候选对象。
更新日期:2020-04-04
中文翻译:

合成,4-氨基-1,2,4-三唑衍生物的体外α-淀粉酶和α-葡萄糖苷酶双重抑制活性及其分子对接和动力学研究。
通过使4-氨基-1,2,4-三唑与多种苯甲醛反应合成了33种4-氨基-1,2,4-三唑衍生物1-33。合成分子通过1 H NMR和EI-MS光谱技术进行表征,并评估其抗高血糖的潜力。与标准阿卡波糖相比,化合物1-33在IC50值范围为2.01±0.03-6.44±0.16和2.09±0.08-6.54±0.10 µM的范围内,具有良好至中等的体外α-淀粉酶和α-葡萄糖苷酶抑制活性。 1.92±0.17 µM)和(IC50 = 1.99±0.07 µM)。有限的结构活性关系表明,合成化合物芳基部分的不同取代是造成可变活性的原因。动力学研究预测,化合物1-33分别受到混合和非竞争性抑制α-淀粉酶和α-葡萄糖苷酶的作用。在计算机研究中,三唑和芳基环以及不同的取代在酶口袋内抑制剂的结合相互作用中起着重要作用。已发现合成分子对两种酶均具有双重抑制潜能,因此它们可作为药物开发和未来研究的主要候选对象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号