当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural modifications of indolinones bearing a pyrrole moiety and discovery of a multi-kinase inhibitor with potent antitumor activity.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.bmc.2020.115486 Mingze Qin 1 , Ye Tian 2 , Xiao Han 3 , Qi Cao 2 , Shuaishuai Zheng 2 , Chunyang Liu 2 , Xia Wu 2 , Lei Liu 2 , Yangyang Meng 2 , Xiaobo Wang 4 , Haotian Zhang 5 , Yunlei Hou 2
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.bmc.2020.115486 Mingze Qin 1 , Ye Tian 2 , Xiao Han 3 , Qi Cao 2 , Shuaishuai Zheng 2 , Chunyang Liu 2 , Xia Wu 2 , Lei Liu 2 , Yangyang Meng 2 , Xiaobo Wang 4 , Haotian Zhang 5 , Yunlei Hou 2
Affiliation
|
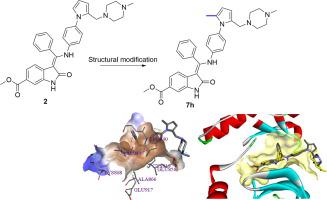
Structural modifications of compound 2, an angiokinase inhibitor reported by our group were performed, which led to the discovery of methyl (Z)-3-(((4-(2-methyl-5-((4-methylpiperazin-1-yl)methyl)-1H-pyrrol-1-yl)phenyl)amino)(phenyl)methylene)-2-oxoindoline-6-carboxylate (7h). Compound 7h exhibited excellent inhibitory activity against angiokinases including VEGFR-1/2/3, PDGFRα/β, and FGFR-1, as well as LYN and c-KIT kinases. At the cellular level, compound 7h significantly attenuated phosphorylation of AKT and ERK proteins, potently inhibited colony formation of HT-29, MKN74, and HepG2 cancer cells, and induced cell apoptosis. Upon incubation with human liver microsome, 7h exhibited comparable metabolic stability to nintedanib. Compound 7h has emerged as a promising lead compound for future drug design.
中文翻译:

带有吡咯部分的吲哚满酮的结构修饰以及发现具有有效抗肿瘤活性的多激酶抑制剂。
进行了我们小组报告的血管激酶抑制剂化合物2的结构修饰,导致发现了甲基(Z)-3-(((4-(2-甲基-5-((4-甲基哌嗪-1-基)甲基)-1H-吡咯-1-基)苯基)氨基)(苯基)亚甲基)-2-氧代二氢吲哚-6-羧酸盐(7h)。化合物7h对包括VEGFR-1 / 2/3,PDGFRα/β和FGFR-1以及LYN和c-KIT激酶的血管激酶表现出优异的抑制活性。在细胞水平,化合物7h显着减弱AKT和ERK蛋白的磷酸化,有效抑制HT-29,MKN74和HepG2癌细胞的集落形成,并诱导细胞凋亡。与人肝微粒体温育后,7h表现出与nintedanib相当的代谢稳定性。化合物7h已成为未来药物设计的有前途的先导化合物。
更新日期:2020-04-03
中文翻译:

带有吡咯部分的吲哚满酮的结构修饰以及发现具有有效抗肿瘤活性的多激酶抑制剂。
进行了我们小组报告的血管激酶抑制剂化合物2的结构修饰,导致发现了甲基(Z)-3-(((4-(2-甲基-5-((4-甲基哌嗪-1-基)甲基)-1H-吡咯-1-基)苯基)氨基)(苯基)亚甲基)-2-氧代二氢吲哚-6-羧酸盐(7h)。化合物7h对包括VEGFR-1 / 2/3,PDGFRα/β和FGFR-1以及LYN和c-KIT激酶的血管激酶表现出优异的抑制活性。在细胞水平,化合物7h显着减弱AKT和ERK蛋白的磷酸化,有效抑制HT-29,MKN74和HepG2癌细胞的集落形成,并诱导细胞凋亡。与人肝微粒体温育后,7h表现出与nintedanib相当的代谢稳定性。化合物7h已成为未来药物设计的有前途的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号