当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Poly(ethylene glycol)–Poly(beta-amino ester)-Based Nanoparticles for Suicide Gene Therapy Enhance Brain Penetration and Extend Survival in a Preclinical Human Glioblastoma Orthotopic Xenograft Model
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-04-06 , DOI: 10.1021/acsbiomaterials.0c00116 Jayoung Kim 1, 2 , Sujan K Mondal 3 , Stephany Y Tzeng 1, 2 , Yuan Rui 1, 2 , Rawan Al-Kharboosh 3 , Kristen K Kozielski 1, 2, 4 , Adip G Bhargav 3, 5 , Cesar A Garcia 3 , Alfredo Quiñones-Hinojosa 3 , Jordan J Green 1, 2, 6, 7, 8
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-04-06 , DOI: 10.1021/acsbiomaterials.0c00116 Jayoung Kim 1, 2 , Sujan K Mondal 3 , Stephany Y Tzeng 1, 2 , Yuan Rui 1, 2 , Rawan Al-Kharboosh 3 , Kristen K Kozielski 1, 2, 4 , Adip G Bhargav 3, 5 , Cesar A Garcia 3 , Alfredo Quiñones-Hinojosa 3 , Jordan J Green 1, 2, 6, 7, 8
Affiliation
|
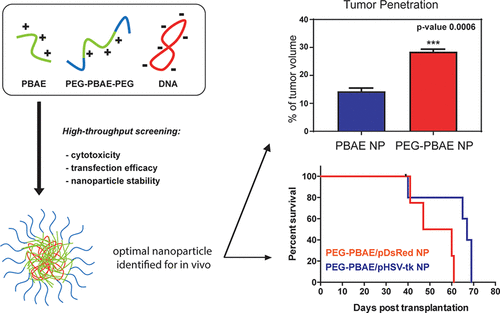
Glioblastoma (GBM) is the most devastating brain cancer, and cures remain elusive with currently available neurosurgical, pharmacological, and radiation approaches. While retrovirus- and adenovirus-mediated suicide gene therapy using DNA encoding herpes simplex virus-thymidine kinase (HSV-tk) and prodrug ganciclovir has been suggested as a promising strategy, a nonviral approach for treatment in an orthotopic human primary brain tumor model has not previously been demonstrated. Delivery challenges include nanoparticle penetration through brain tumors, efficient cancer cell uptake, endosomal escape to the cytosol, and biodegradability. To meet these challenges, we synthesized poly(ethylene glycol)–modified poly(beta-amino ester) (PEG–PBAE) polymers to improve extracellular delivery and coencapsulated plasmid DNA with end-modified poly(beta-amino ester) (ePBAE) polymers to improve intracellular delivery as well. We created and evaluated a library of PEG–PBAE/ePBAE nanoparticles (NPs) for effective gene therapy against two independent primary human stem-like brain tumor initiating cells, a putative target to prevent GBM recurrence. The optimally engineered PEG–PBAE/ePBAE NP formulation demonstrated 54 and 82% transfection efficacies in GBM1A and BTIC375 cells respectively, in comparison to 37 and 66% for optimized PBAE NPs without PEG. The leading PEG–PBAE NP formulation also maintained sub-250 nm particle size up to 5 h, while PBAE NPs without PEG showed aggregation over time to micrometer-sized complexes. The comparative advantage demonstrated in vitro successfully translated into improved in vivo diffusion, with a higher amount of PEG–PBAE NPs penetrating to a distance of 2 mm from the injection site. A significant increase in median survival from 53.5 to 67 days by PEG–PBAE/pHSV-tk NP and systemic ganciclovir treatment compared to a control group in orthotopic murine model of human glioblastoma demonstrates the potential of PEG–PBAE-based NPs as an effective gene therapy platform for the treatment of human brain tumors.
中文翻译:

用于自杀基因治疗的聚(乙二醇)-聚(β-氨基酯)纳米颗粒可增强临床前人胶质母细胞瘤原位异种移植模型中的大脑渗透并延长生存期
胶质母细胞瘤 (GBM) 是最具破坏性的脑癌,目前可用的神经外科、药物和放射方法仍难以治愈。虽然使用编码单纯疱疹病毒胸苷激酶 (HSV-tk) 的 DNA 和前药更昔洛韦的逆转录病毒和腺病毒介导的自杀基因治疗被认为是一种有前途的策略,但在原位人类原发性脑肿瘤模型中使用非病毒治疗方法尚未出现。之前已被证明。递送挑战包括纳米颗粒穿透脑肿瘤、癌细胞的有效摄取、内体逃逸至细胞质以及生物降解性。为了应对这些挑战,我们合成了聚(乙二醇)修饰的聚(β-氨基酯)(PEG-PBAE)聚合物以改善细胞外递送,并用末端修饰的聚(β-氨基酯)(ePBAE)聚合物共封装质粒DNA还可以改善细胞内递送。我们创建并评估了 PEG-PBAE/ePBAE 纳米颗粒 (NP) 库,用于针对两种独立的原发性人类干细胞样脑肿瘤起始细胞(预防 GBM 复发的假定目标)进行有效的基因治疗。优化设计的 PEG-PBAE/ePBAE NP 制剂在 GBM1A 和 BTIC375 细胞中的转染效率分别为 54% 和 82%,而不含 PEG 的优化 PBAE NP 的转染效率分别为 37% 和 66%。领先的 PEG-PBAE NP 配方还可在长达 5 小时内保持低于 250 nm 的粒径,而不含 PEG 的 PBAE NP 随着时间的推移会聚集成微米大小的复合物。体外证明的比较优势成功转化为体内扩散的改善,更多的 PEG-PBAE NP 渗透到距注射部位 2 毫米的距离。在人胶质母细胞瘤原位小鼠模型中,与对照组相比,PEG-PBAE/pHSV-tk NP 和全身更昔洛韦治疗的中位生存期从 53.5 天显着增加至 67 天,这表明基于 PEG-PBAE 的 NP 作为有效基因的潜力用于治疗人类脑肿瘤的治疗平台。
更新日期:2020-04-06
中文翻译:

用于自杀基因治疗的聚(乙二醇)-聚(β-氨基酯)纳米颗粒可增强临床前人胶质母细胞瘤原位异种移植模型中的大脑渗透并延长生存期
胶质母细胞瘤 (GBM) 是最具破坏性的脑癌,目前可用的神经外科、药物和放射方法仍难以治愈。虽然使用编码单纯疱疹病毒胸苷激酶 (HSV-tk) 的 DNA 和前药更昔洛韦的逆转录病毒和腺病毒介导的自杀基因治疗被认为是一种有前途的策略,但在原位人类原发性脑肿瘤模型中使用非病毒治疗方法尚未出现。之前已被证明。递送挑战包括纳米颗粒穿透脑肿瘤、癌细胞的有效摄取、内体逃逸至细胞质以及生物降解性。为了应对这些挑战,我们合成了聚(乙二醇)修饰的聚(β-氨基酯)(PEG-PBAE)聚合物以改善细胞外递送,并用末端修饰的聚(β-氨基酯)(ePBAE)聚合物共封装质粒DNA还可以改善细胞内递送。我们创建并评估了 PEG-PBAE/ePBAE 纳米颗粒 (NP) 库,用于针对两种独立的原发性人类干细胞样脑肿瘤起始细胞(预防 GBM 复发的假定目标)进行有效的基因治疗。优化设计的 PEG-PBAE/ePBAE NP 制剂在 GBM1A 和 BTIC375 细胞中的转染效率分别为 54% 和 82%,而不含 PEG 的优化 PBAE NP 的转染效率分别为 37% 和 66%。领先的 PEG-PBAE NP 配方还可在长达 5 小时内保持低于 250 nm 的粒径,而不含 PEG 的 PBAE NP 随着时间的推移会聚集成微米大小的复合物。体外证明的比较优势成功转化为体内扩散的改善,更多的 PEG-PBAE NP 渗透到距注射部位 2 毫米的距离。在人胶质母细胞瘤原位小鼠模型中,与对照组相比,PEG-PBAE/pHSV-tk NP 和全身更昔洛韦治疗的中位生存期从 53.5 天显着增加至 67 天,这表明基于 PEG-PBAE 的 NP 作为有效基因的潜力用于治疗人类脑肿瘤的治疗平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号