当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of β-Phenethylamines via Ni/Photoredox Cross-Electrophile Coupling of Aliphatic Aziridines and Aryl Iodides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-06 , DOI: 10.1021/jacs.0c01724 Talia J Steiman 1 , Junyi Liu 1 , Amanuella Mengiste 1 , Abigail G Doyle 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-06 , DOI: 10.1021/jacs.0c01724 Talia J Steiman 1 , Junyi Liu 1 , Amanuella Mengiste 1 , Abigail G Doyle 1
Affiliation
|
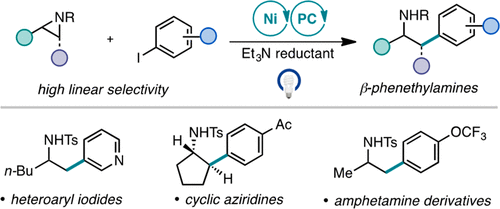
A photoassisted Ni-catalyzed reductive cross-coupling between tosyl-protected alkyl aziridines and commercially available (hetero)aryl iodides is reported. This mild and modular method proceeds in the absence of stoichiometric heterogeneous reductants and uses an inexpensive organic photocatalyst to access medicinally valuable β-phenethylamine derivatives. Unprecedented reactivity was achieved with the activation of cyclic aziridines. Mechanistic studies suggest that the regioselectivity and reactivity observed under these conditions are a result of nucleophilic iodide ring opening of the aziridine to generate an iodoamine as the active electrophile. This strategy also enables cross-coupling with Boc-protected aziridines.
中文翻译:

通过脂肪族氮丙啶和芳基碘的 Ni/Photoredox 交叉电偶偶联合成 β-苯乙胺
报道了甲苯磺酰基保护的烷基氮丙啶和市售(杂)芳基碘化物之间的光辅助镍催化还原交叉偶联。这种温和且模块化的方法在没有化学计量的多相还原剂的情况下进行,并使用廉价的有机光催化剂来获得具有药用价值的 β-苯乙胺衍生物。环氮丙啶的活化实现了前所未有的反应性。机理研究表明,在这些条件下观察到的区域选择性和反应性是氮丙啶亲核碘化物开环生成碘胺作为活性亲电子试剂的结果。该策略还能够与 Boc 保护的氮丙啶交叉偶联。
更新日期:2020-04-06
中文翻译:

通过脂肪族氮丙啶和芳基碘的 Ni/Photoredox 交叉电偶偶联合成 β-苯乙胺
报道了甲苯磺酰基保护的烷基氮丙啶和市售(杂)芳基碘化物之间的光辅助镍催化还原交叉偶联。这种温和且模块化的方法在没有化学计量的多相还原剂的情况下进行,并使用廉价的有机光催化剂来获得具有药用价值的 β-苯乙胺衍生物。环氮丙啶的活化实现了前所未有的反应性。机理研究表明,在这些条件下观察到的区域选择性和反应性是氮丙啶亲核碘化物开环生成碘胺作为活性亲电子试剂的结果。该策略还能够与 Boc 保护的氮丙啶交叉偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号