当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Dimerization Properties of Cup‐ and Bowl‐shaped Cyclic Trilactams
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-04-01 , DOI: 10.1002/ajoc.202000140 Sitanan Sartyoungkul 1 , Yumi Yakiyama 1 , Hidehiro Sakurai 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-04-01 , DOI: 10.1002/ajoc.202000140 Sitanan Sartyoungkul 1 , Yumi Yakiyama 1 , Hidehiro Sakurai 1
Affiliation
|
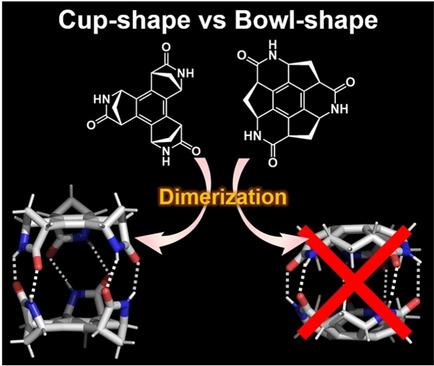
Two chiral trilactams, cup‐shaped (−)‐1 and bowl‐shaped (−)‐2 , were synthesized. Single crystal X‐ray analysis revealed that (−)‐1 formed dimer structure by the formation of three NH⋅⋅⋅O type complementary hydrogen bonds at three amide moieties. The equilibrium constant (K eq) and kinetic parameters ▵H ° and ▵S ° of the dimer formation phenomenon of (−)‐1 were determined by variable temperature proton NMR. Further binding energy calculation on both (−)‐1 and (−)‐2 dimers confirmed the more favourable formation of (−)‐1 dimer than (−)‐2 dimer, explaining the significant solubility difference of the two against the less polar solvent, such as CHCl3. Trilactam (−)‐1 afforded coordination polymer by complexation with Zn2+, keeping the capsule‐like structure.
中文翻译:

杯形和碗形环状三内酰胺的合成和二聚性质
合成了两个手性三内酰胺,杯形(-)- 1和碗形(-)- 2。单晶X射线分析表明(-)- 1通过在三个酰胺部分形成三个NH····O型互补氢键形成二聚体结构。平衡常数(ķ当量)和动力学参数▵ ħ °和▵小号的二聚体形成现象°( - ) - 1,通过变温质子NMR测定。对(−)- 1和(−)‐ 2二聚体的进一步结合能计算证实了(−)‐ 1二聚体比(−)‐ 2更有利的形成二聚体,解释了两者相对于极性较小的溶剂(如CHCl 3)的显着溶解度差异。Trilactam(-)- 1通过与Zn 2+络合提供配位聚合物,保持了胶囊状结构。
更新日期:2020-04-01
中文翻译:

杯形和碗形环状三内酰胺的合成和二聚性质
合成了两个手性三内酰胺,杯形(-)- 1和碗形(-)- 2。单晶X射线分析表明(-)- 1通过在三个酰胺部分形成三个NH····O型互补氢键形成二聚体结构。平衡常数(ķ当量)和动力学参数▵ ħ °和▵小号的二聚体形成现象°( - ) - 1,通过变温质子NMR测定。对(−)- 1和(−)‐ 2二聚体的进一步结合能计算证实了(−)‐ 1二聚体比(−)‐ 2更有利的形成二聚体,解释了两者相对于极性较小的溶剂(如CHCl 3)的显着溶解度差异。Trilactam(-)- 1通过与Zn 2+络合提供配位聚合物,保持了胶囊状结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号