当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Evaluation of Pollutants in the Environment: Interaction Between the Metal Ions Zn(II) and Cu(II) with the Fungicide Thiram in Billings Dam
Electroanalysis ( IF 2.7 ) Pub Date : 2020-03-27 , DOI: 10.1002/elan.201900438 Vitor Alves Sá da Silva 1 , Aymara Silva Santos 1 , Tiago Luiz Ferreira 1 , Lúcia Codognoto 1 , Eliana Maíra Agostini Valle 1
Electroanalysis ( IF 2.7 ) Pub Date : 2020-03-27 , DOI: 10.1002/elan.201900438 Vitor Alves Sá da Silva 1 , Aymara Silva Santos 1 , Tiago Luiz Ferreira 1 , Lúcia Codognoto 1 , Eliana Maíra Agostini Valle 1
Affiliation
|
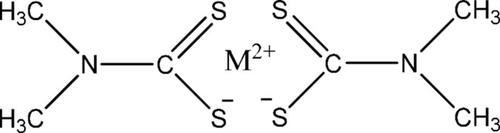
Pesticides are organic molecules used in the control of various pests in different crops. These molecules show functional groups that can interact with metal ions, forming new species with different properties. These new compounds have been attracting attention because they can become a new environmental problem. In this work the interaction of copper and zinc metal ions with Thiram pesticide was studied using electrochemical techniques. Studies in ultrapure water showed the formation of Zn−Thiram complex with reduction potential at −1.330 V; Cu−Thiram complex showed a cathodic peak at 0.020 V. Thiram causes a different effect on the two metal ions studied. It was observed that the ligand stabilizes more the Cu(II) than Zn(II). Both systems proved to be quasi‐reversible, controlled by the adsorption of the species on the electrode surface. The formation constants of the complexes were calculated to be 2.1×105 for Zn−Thiram and 1.5×1019 for Cu−Thiram. In the samples from Billings dam, the Zn‐complex showed reduction potential at −1.403 V; Cu‐complex exhibited a reduction peak at 0.012 V. Although there are interferers in river waters, the interaction of these metals with the pesticide showed high affinity, being possible to detect them in natural samples. The Cu(II) complex showed to be more stable in natural matrices when compared to the Zn(II) complex. The sensitivity for thiram electroanalytical determination decreases in the presence of Zn(II) and Cu(II).
中文翻译:

电化学评估环境中的污染物:比林斯大坝中金属离子Zn(II)和Cu(II)与杀菌剂Thiram的相互作用
农药是用于控制不同农作物中各种害虫的有机分子。这些分子显示出可以与金属离子相互作用的官能团,从而形成了具有不同特性的新物种。这些新化合物已引起人们的关注,因为它们可能会成为新的环境问题。在这项工作中,使用电化学技术研究了铜和锌金属离子与Thiram农药的相互作用。在超纯水中的研究表明,Zn-Thiram络合物的形成在-1.330 V时具有还原电势。Cu-Thiram络合物在0.020 V处显示一个阴极峰。Thiram对所研究的两种金属离子产生不同的影响。观察到,配体比Zn(II)更稳定Cu(II)。两种系统都被证明是准系统可逆的,受物质在电极表面的吸附控制。对于Zn-Thiram,络合物的形成常数经计算为2.1×10 5,对于Cu-Thiram,络合物的形成常数为1.5×10 19。在比林斯大坝的样品中,锌复合物在-1.403 V时显示出还原电位;铜络合物在0.012 V处显示出还原峰。尽管河水中存在干扰物,但这些金属与农药的相互作用显示出很高的亲和力,可以在天然样品中检测到。与Zn(II)配合物相比,Cu(II)配合物在天然基质中表现出更稳定的性能。在存在Zn(II)和Cu(II)的情况下,对锡拉姆电分析测定的灵敏度降低。
更新日期:2020-03-27
中文翻译:

电化学评估环境中的污染物:比林斯大坝中金属离子Zn(II)和Cu(II)与杀菌剂Thiram的相互作用
农药是用于控制不同农作物中各种害虫的有机分子。这些分子显示出可以与金属离子相互作用的官能团,从而形成了具有不同特性的新物种。这些新化合物已引起人们的关注,因为它们可能会成为新的环境问题。在这项工作中,使用电化学技术研究了铜和锌金属离子与Thiram农药的相互作用。在超纯水中的研究表明,Zn-Thiram络合物的形成在-1.330 V时具有还原电势。Cu-Thiram络合物在0.020 V处显示一个阴极峰。Thiram对所研究的两种金属离子产生不同的影响。观察到,配体比Zn(II)更稳定Cu(II)。两种系统都被证明是准系统可逆的,受物质在电极表面的吸附控制。对于Zn-Thiram,络合物的形成常数经计算为2.1×10 5,对于Cu-Thiram,络合物的形成常数为1.5×10 19。在比林斯大坝的样品中,锌复合物在-1.403 V时显示出还原电位;铜络合物在0.012 V处显示出还原峰。尽管河水中存在干扰物,但这些金属与农药的相互作用显示出很高的亲和力,可以在天然样品中检测到。与Zn(II)配合物相比,Cu(II)配合物在天然基质中表现出更稳定的性能。在存在Zn(II)和Cu(II)的情况下,对锡拉姆电分析测定的灵敏度降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号