当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Halohydroxylation of α,β‐Unsaturated Ketones with Water as the Nucleophile
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1002/adsc.202000080 Weiwei Li 1 , Pengfei Zhou 1 , Gonglin Li 1 , Lili Lin 1 , Xiaoming Feng 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1002/adsc.202000080 Weiwei Li 1 , Pengfei Zhou 1 , Gonglin Li 1 , Lili Lin 1 , Xiaoming Feng 1
Affiliation
|
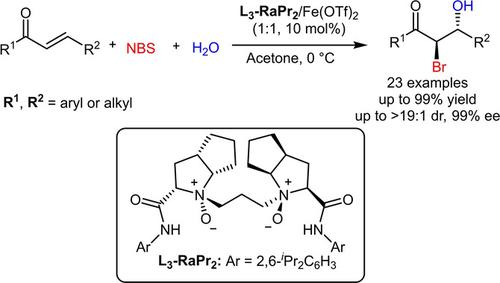
The catalytic asymmetric halohydroxylation of α,β‐unsaturated ketones with water as the nucleophile has been realized by applying a chiral N,N′‐dioxide/Fe(OTf)2 complex as the catalyst. Bromo‐, chloro‐ and iodo‐hydroxylations were all suitable in this catalytic system. A variety of α‐halo‐β‐hydroxy ketones were obtained in often good yields with generally high dr and ee values. Besides, a Michael/α‐halogenation process was considered more possible and a possible transition state model was proposed to explain the origin of stereoselectivity.
中文翻译:

亲核试剂以水催化α,β-不饱和酮的不对称卤代羟基化反应
α的不对称催化halohydroxylation,用水β不饱和酮作为亲核试剂已经通过应用手性实现Ñ,N'二氧化物/铁(OTF)2配合物作为催化剂。溴,氯和碘羟基化反应均适用于该催化体系。通常以高收率获得各种α-卤代-β-羟基酮,且其dr和ee值通常较高。此外,认为迈克尔/α-卤化过程更可能,并提出了可能的过渡态模型来解释立体选择性的起源。
更新日期:2020-04-01
中文翻译:

亲核试剂以水催化α,β-不饱和酮的不对称卤代羟基化反应
α的不对称催化halohydroxylation,用水β不饱和酮作为亲核试剂已经通过应用手性实现Ñ,N'二氧化物/铁(OTF)2配合物作为催化剂。溴,氯和碘羟基化反应均适用于该催化体系。通常以高收率获得各种α-卤代-β-羟基酮,且其dr和ee值通常较高。此外,认为迈克尔/α-卤化过程更可能,并提出了可能的过渡态模型来解释立体选择性的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号