当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand Effects in Calcium Catalyzed Ketone Hydroboration
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-04-30 , DOI: 10.1002/ejic.202000264 Steffen Brand 1 , Andrea Causero 1 , Holger Elsen 1 , Jürgen Pahl 1 , Jens Langer 1 , Sjoerd Harder 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-04-30 , DOI: 10.1002/ejic.202000264 Steffen Brand 1 , Andrea Causero 1 , Holger Elsen 1 , Jürgen Pahl 1 , Jens Langer 1 , Sjoerd Harder 1
Affiliation
|
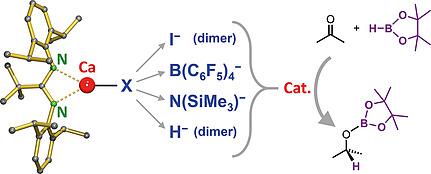
The first “naked” (Lewis base‐free) cationic Ca amidinate complex [tBuAmDIPPCa(C6H6)]+[B(C6F5)4]– was prepared in 62 % yield {tBuAmDIPP = tBuC(N–DIPP)2; DIPP = 2,6‐diisopropylphenyl} by reaction of [tBuAmDIPPCaH]2 with [Ph3C]+[B(C6F5)4]– in chlorobenzene. The ether‐free complex tBuAmDIPPCaN(SiMe3)2 was obtained by removal of diethyl ether from its ether adduct. Crystal structures show that the amidinate ligand in both complexes is N,Aryl‐chelating. In this coordination mode the bulk of the amidinate ligand is comparable to that of a DIPP‐substituted β‐diketiminate ligand. Isomers with N,N‐coordinating amidinate ligands are circa 15 kcal/mol higher in energy and this coordination mode is only present in case additional ether ligands compensate for energy loss or in case of space limitation at the metal, e.g. in homoleptic (tBuAmDIPP)2Ca. A series of four Ca amidinate complexes, tBuAmDIPPCaX, were tested in the catalytic hydroboration of ketones and aldehydes by pinacolborane (HBpin). Catalytic activities increase for X– = I– < B(C6F5)4– < (Me3Si)2N– ≈ H–. For catalysts with unreactive anions, like I– or B(C6F5)4–, catalyst performance increases with the Lewis acidity of the metal and a mechanism is proposed in which HBpin and ketone coordinate to the Ca2+ ion which is followed by direct hydroboration. The more active catalysts with X– = (Me3Si)2N– or H– likely operate through a mechanism which involves intermediate metal hydride (or borate) complexes.
中文翻译:

钙催化酮硼氢化反应中的配体效应
制备了第一个“裸露”(无路易斯碱的)阳离子Ca胺酸络合物[ t Bu Am DIPP Ca(C 6 H 6)] + [B(C 6 F 5)4 ] –收率为{ t Bu AmDIPP =t BuC(N–DIPP)2;通过[ t Bu Am DIPP CaH] 2与[Ph 3 C] + [B(C 6 F 5)4 ]反应使DIPP = 2,6-二异丙基苯基} –在氯苯中。通过从乙醚加合物中除去乙醚,获得了无醚配合物t Bu Am DIPP CaN(SiMe 3)2。晶体结构表明两种配合物中的mid基配体都是N,芳基螯合的。在这种配位模式下,the酰胺配体的体积可与DIPP取代的β-二酮胺配体的体积相媲美。具有N,N配位的mid酰胺配体的异构体的能量高约15 kcal / mol,只有在其他醚配体补偿能量损失或金属空间受限的情况下,例如在均化剂(t Bu上午DIPP)2钙 在频哪醇硼烷(HBpin)催化的酮和醛加氢硼化反应中,测试了一系列的四个Ca胺酸钙络合物t Bu Am DIPP CaX。催化活性增加对X - = I - <B(C 6 ˚F 5)4 - <(ME 3 Si)的2 ñ - ≈ħ - 。对于具有非反应性阴离子的催化剂,例如I –或B(C 6 F 5)4 –,催化剂性能随金属的路易斯酸度增加而增加,并提出了一种机理,其中HBpin和酮与Ca 2+离子配位,然后直接进行硼氢化。与X更具活性的催化剂- =(ME 3 Si)的2 ñ -或H -可能通过其涉及中间金属氢化物(或硼酸盐)配合的机构操作。
更新日期:2020-04-30
中文翻译:

钙催化酮硼氢化反应中的配体效应
制备了第一个“裸露”(无路易斯碱的)阳离子Ca胺酸络合物[ t Bu Am DIPP Ca(C 6 H 6)] + [B(C 6 F 5)4 ] –收率为{ t Bu AmDIPP =t BuC(N–DIPP)2;通过[ t Bu Am DIPP CaH] 2与[Ph 3 C] + [B(C 6 F 5)4 ]反应使DIPP = 2,6-二异丙基苯基} –在氯苯中。通过从乙醚加合物中除去乙醚,获得了无醚配合物t Bu Am DIPP CaN(SiMe 3)2。晶体结构表明两种配合物中的mid基配体都是N,芳基螯合的。在这种配位模式下,the酰胺配体的体积可与DIPP取代的β-二酮胺配体的体积相媲美。具有N,N配位的mid酰胺配体的异构体的能量高约15 kcal / mol,只有在其他醚配体补偿能量损失或金属空间受限的情况下,例如在均化剂(t Bu上午DIPP)2钙 在频哪醇硼烷(HBpin)催化的酮和醛加氢硼化反应中,测试了一系列的四个Ca胺酸钙络合物t Bu Am DIPP CaX。催化活性增加对X - = I - <B(C 6 ˚F 5)4 - <(ME 3 Si)的2 ñ - ≈ħ - 。对于具有非反应性阴离子的催化剂,例如I –或B(C 6 F 5)4 –,催化剂性能随金属的路易斯酸度增加而增加,并提出了一种机理,其中HBpin和酮与Ca 2+离子配位,然后直接进行硼氢化。与X更具活性的催化剂- =(ME 3 Si)的2 ñ -或H -可能通过其涉及中间金属氢化物(或硼酸盐)配合的机构操作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号