当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd/TiC/Ti electrode with enhanced atomic H* generation, atomic H* adsorption and 2,4-DCBA adsorption for facilitating electrocatalytic hydrodechlorination
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-04-03 , DOI: 10.1039/d0en00182a Zimo Lou 1, 2, 3, 4 , Zheni Wang 1, 2, 3, 4 , Jiasheng Zhou 1, 2, 3, 4 , Chuchen Zhou 1, 2, 3, 4 , Jiang Xu 5, 6, 7, 8 , Xinhua Xu 1, 2, 3, 4
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-04-03 , DOI: 10.1039/d0en00182a Zimo Lou 1, 2, 3, 4 , Zheni Wang 1, 2, 3, 4 , Jiasheng Zhou 1, 2, 3, 4 , Chuchen Zhou 1, 2, 3, 4 , Jiang Xu 5, 6, 7, 8 , Xinhua Xu 1, 2, 3, 4
Affiliation
|
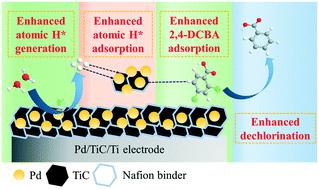
Electrocatalytic hydrodechlorination (ECH) has been proposed as a potential technology for the effective degradation of organochlorine contaminants. In this study, a nanoscale Pd/TiC catalyst with low resistance was dip-coated onto Ti, aiming to fabricate a stable Pd/TiC/Ti electrode with enhanced ECH reactivity for efficient dechlorination. Electrochemical techniques indicated that the Pd/TiC/Ti electrode had a larger electrochemically active surface area and more rapid interfacial charge transfer than the conventional Pd/C/Ti electrode. In a batch experiment, the dechlorination rate for ECH of 2,4-dichlorobenzoic acid (2,4-DCBA, a model pollutant) by Pd/TiC/Ti was 1.3–14.5 times higher than those by Pd/C/Ti, Pd/MWCNTs/Ti, and Pd/MoS2/Ti, respectively. Scavenger experiments, ESR spin-trapping spectroscopy, and cyclic voltammetry clarified the intensified atomic H* generation by Pd/TiC/Ti. DFT calculations revealed that Pd/TiC/Ti had stronger binding ability with atomic H* (Eads: −2.89 eV vs. −2.44 eV) and 2,4-DCBA (Eads: −3.62 eV vs. −2.15 eV), respectively, when compared with Pd/C/Ti. As a result, enhanced generation of atomic H*, together with strengthened adsorption of atomic H* and 2,4-DCBA, would contribute to a faster ECH reaction. The Pd/TiC/Ti electrode retained its reactivity after 10 successive batch experiments (80 h), and showed high tolerance to the constituents in actual water matrices including lake water and river water. This study proposed a promising ECH electrode for the treatment of chlorinated organics in water matrices.
中文翻译:

具有增强的原子H *生成,原子H *吸附和2,4-DCBA吸附的Pd / TiC / Ti电极,可促进电催化加氢脱氯
已经提出电催化加氢脱氯(ECH)作为有效降解有机氯污染物的潜在技术。在这项研究中,将具有低电阻的纳米级Pd / TiC催化剂浸涂到Ti上,旨在制造具有增强的ECH反应性的稳定Pd / TiC / Ti电极,以进行有效的脱氯。电化学技术表明,Pd / TiC / Ti电极比常规Pd / C / Ti电极具有更大的电化学活性表面积和更快的界面电荷转移。在分批实验中,Pd / TiC / Ti对2,4-二氯苯甲酸(2,4-DCBA,一种典型污染物)的ECH脱氯速率比Pd / C / Ti,Pd的脱氯速率高1.3–14.5倍/ MWCNTs / Ti和Pd / MoS 2/ Ti。清除剂实验,ESR自旋阱光谱和循环伏安法阐明了Pd / TiC / Ti增强了原子H *的产生。DFT计算显示,Pd / TiC / Ti与原子H *(E ads:-2.89 eV vs.- 2.44 eV)和2,4-DCBA(E ads:-3.62 eV vs.与Pd / C / Ti相比,分别为-2.15 eV)。结果,增强的原子H *的生成以及增强的原子H *和2,4-DCBA的吸附将有助于更快的ECH反应。Pd / TiC / Ti电极在连续10次分批实验(80小时)后仍保持其反应活性,并且对包括湖泊水和河水在内的实际水基质中的成分表现出较高的耐受性。这项研究提出了一种有前途的ECH电极,用于处理水基质中的氯化有机物。
更新日期:2020-04-03
中文翻译:

具有增强的原子H *生成,原子H *吸附和2,4-DCBA吸附的Pd / TiC / Ti电极,可促进电催化加氢脱氯
已经提出电催化加氢脱氯(ECH)作为有效降解有机氯污染物的潜在技术。在这项研究中,将具有低电阻的纳米级Pd / TiC催化剂浸涂到Ti上,旨在制造具有增强的ECH反应性的稳定Pd / TiC / Ti电极,以进行有效的脱氯。电化学技术表明,Pd / TiC / Ti电极比常规Pd / C / Ti电极具有更大的电化学活性表面积和更快的界面电荷转移。在分批实验中,Pd / TiC / Ti对2,4-二氯苯甲酸(2,4-DCBA,一种典型污染物)的ECH脱氯速率比Pd / C / Ti,Pd的脱氯速率高1.3–14.5倍/ MWCNTs / Ti和Pd / MoS 2/ Ti。清除剂实验,ESR自旋阱光谱和循环伏安法阐明了Pd / TiC / Ti增强了原子H *的产生。DFT计算显示,Pd / TiC / Ti与原子H *(E ads:-2.89 eV vs.- 2.44 eV)和2,4-DCBA(E ads:-3.62 eV vs.与Pd / C / Ti相比,分别为-2.15 eV)。结果,增强的原子H *的生成以及增强的原子H *和2,4-DCBA的吸附将有助于更快的ECH反应。Pd / TiC / Ti电极在连续10次分批实验(80小时)后仍保持其反应活性,并且对包括湖泊水和河水在内的实际水基质中的成分表现出较高的耐受性。这项研究提出了一种有前途的ECH电极,用于处理水基质中的氯化有机物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号