当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of structure-directing polyhedra and heterocyclic ligands on the chain structures and O/F ordering in a series of zinc vanadium oxyfluorides
CrystEngComm ( IF 2.6 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0ce00318b Belal Ahmed 1, 2, 3, 4 , Hongil Jo 1, 4, 5, 6 , Suheon Lee 2, 4, 7 , Kwang Yong Choi 2, 4, 7 , Kang Min Ok 1, 4, 5, 6
CrystEngComm ( IF 2.6 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0ce00318b Belal Ahmed 1, 2, 3, 4 , Hongil Jo 1, 4, 5, 6 , Suheon Lee 2, 4, 7 , Kwang Yong Choi 2, 4, 7 , Kang Min Ok 1, 4, 5, 6
Affiliation
|
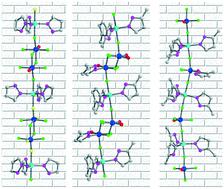
Three transition metal oxyfluorides consisting of distinctive structure-directing vanadium oxyfluoride polyhedra and highly polarizable Zn2+ cations, [Zn(pz)2][Zn(pz)3][V2O2F8] (1, pz = pyrazole), [Zn(mpz)3][V2O2F6(H2O)(mpz)] (2, mpz = 3-methylpyrazole), and [Zn(mpz)3]3[VOF4(mpz)][VOF4]2 (3), were synthesized by mild hydrothermal reactions at 150 °C. Compound 1 reveals a layered structure with zigzag chains, in which [Zn(pz)2/3]2+ cations and dimeric [V2O2F8]4− anions are combined in both cis- and trans-coordinating modes. The trans-directing dimeric [V2O2F6(H2O)(mpz)] and monomeric [VOF4(mpz)] and [VOF4] units direct the linear chain structures of compounds 2 and 3. The void space found in compound 2 is attributable to the interchain hydrogen-bonding networks. The observed absorption band gaps of 3.21–4.25 eV for the reported compounds originate from the octahedral distortion of Zn2+ cations and the interorbital electronic transitions. The reduction of V5+ to V4+ in acidic media might occur via a free-radical mechanism, where the dioxovanadium(V) undergoes a one-electron reduction by pyrazole and 3-methylpyrazole. Complete characterization including thermogravimetric analysis, spectroscopy, and dipole moment calculations is provided.
中文翻译:

定向结构多面体和杂环配体对一系列氟锌钒氧化物链结构和O / F有序性的影响
三种过渡金属氟氧化物,由结构独特的氟氧化钒多面体和高度极化的Zn 2+阳离子[Zn(pz)2 ] [Zn(pz)3 ] [V 2 O 2 F 8 ](1,pz =吡唑)组成,[Zn(mpz)3 ] [V 2 O 2 F 6(H 2 O)(mpz)](2,mpz = 3-甲基吡唑)和[Zn(mpz)3 ] 3 [VOF 4(mpz)] [VOF 4 ] 2(3)是在150°C下通过温和的水热反应合成的。化合物1揭示的层状结构与锯齿链,其中[锌(PZ)2/3 ] 2+阳离子和二聚[V 2 ö 2 ˚F 8 ] 4-阴离子被结合在两个顺式-和反式-coordinating模式。所述反式-directing二聚体[V 2 ö 2 ˚F 6(H 2 O)(MPZ)]和单体[VOF 4(MPZ)]和[VOF 4 ]单位的直接化合物的直链结构2和3。在化合物2中发现的空隙空间可归因于链间氢键网络。所报告的化合物观察到的吸收带隙为3.21-4.25 eV,这是由于Zn 2+阳离子的八面体形变和轨道间电子跃迁引起的。在酸性介质中V 5+还原为V 4+可能是通过自由基机理发生的,其中二恶钒钒(V)受到吡唑和3-甲基吡唑的单电子还原。提供了完整的特性描述,包括热重分析,光谱学和偶极矩计算。
更新日期:2020-04-02
中文翻译:

定向结构多面体和杂环配体对一系列氟锌钒氧化物链结构和O / F有序性的影响
三种过渡金属氟氧化物,由结构独特的氟氧化钒多面体和高度极化的Zn 2+阳离子[Zn(pz)2 ] [Zn(pz)3 ] [V 2 O 2 F 8 ](1,pz =吡唑)组成,[Zn(mpz)3 ] [V 2 O 2 F 6(H 2 O)(mpz)](2,mpz = 3-甲基吡唑)和[Zn(mpz)3 ] 3 [VOF 4(mpz)] [VOF 4 ] 2(3)是在150°C下通过温和的水热反应合成的。化合物1揭示的层状结构与锯齿链,其中[锌(PZ)2/3 ] 2+阳离子和二聚[V 2 ö 2 ˚F 8 ] 4-阴离子被结合在两个顺式-和反式-coordinating模式。所述反式-directing二聚体[V 2 ö 2 ˚F 6(H 2 O)(MPZ)]和单体[VOF 4(MPZ)]和[VOF 4 ]单位的直接化合物的直链结构2和3。在化合物2中发现的空隙空间可归因于链间氢键网络。所报告的化合物观察到的吸收带隙为3.21-4.25 eV,这是由于Zn 2+阳离子的八面体形变和轨道间电子跃迁引起的。在酸性介质中V 5+还原为V 4+可能是通过自由基机理发生的,其中二恶钒钒(V)受到吡唑和3-甲基吡唑的单电子还原。提供了完整的特性描述,包括热重分析,光谱学和偶极矩计算。











































 京公网安备 11010802027423号
京公网安备 11010802027423号