当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of (-)-solanidine and (-)-tomatidenol.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0ob00457j Yun Wang 1 , Guanxin Huang 2 , Yong Shi 3 , Wei-Sheng Tian 3 , Chunlin Zhuang 1 , Fen-Er Chen 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0ob00457j Yun Wang 1 , Guanxin Huang 2 , Yong Shi 3 , Wei-Sheng Tian 3 , Chunlin Zhuang 1 , Fen-Er Chen 4
Affiliation
|
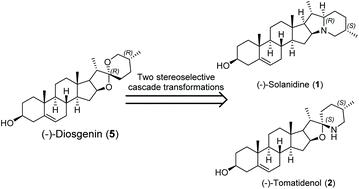
A concise asymmetric synthesis of two naturally occurring seco-type cholestane alkaloids (-)-solanidine and (-)-tomatidenol from (-)-diosgenin with a linear reaction sequence of 12 steps and 13 steps, respectively, is reported. The synthetic strategy includes the highly controlled establishment of highly functionalized octahydroindolizine ((-)-solanidine) and 1-oxa-6-azaspiro[4.5]decane ((-)-tomatidenol) cores with five stereocenters, respectively, from (-)-diosgenin, featuring two stereoselective cascade transformations including a modified cascade ring-switching process of furostan-26-acid to open the E-ring of (-)-diosgenin and a cascade azide reduction/intramolecular reductive amination to close the E- and F-rings of (-)-solanidine and (-)-tomatidenol. This work should enable further explorations of chemical and biological spaces based on solanidine, tomatidenol and related natural products.
中文翻译:

(-)-茄啶和(-)-番茄甾醇的不对称合成。
据报道,从(-)-薯s皂苷元中线性合成的两种山高胆固醇型胆甾烷生物碱(-)-茄碱和(-)-番茄甾醇的简明不对称合成反应的线性反应顺序分别为12步和13步。合成策略包括高度受控地从(-)-分别建立具有五个立体中心的高度官能化的八氢吲哚嗪((-)-茄啶)和1-oxa-6-氮杂螺[4.5]癸烷((-)-番茄甾醇)核。薯gen皂素,具有两个立体选择性的级联转化,包括改良的呋喃斯坦-26-酸级联环转换过程以打开(-)-薯-皂苷元的E环,以及叠氮化物还原/分子内还原胺化以关闭E-和F- (-)-茄啶和(-)-番茄甾醇的环。
更新日期:2020-04-02
中文翻译:

(-)-茄啶和(-)-番茄甾醇的不对称合成。
据报道,从(-)-薯s皂苷元中线性合成的两种山高胆固醇型胆甾烷生物碱(-)-茄碱和(-)-番茄甾醇的简明不对称合成反应的线性反应顺序分别为12步和13步。合成策略包括高度受控地从(-)-分别建立具有五个立体中心的高度官能化的八氢吲哚嗪((-)-茄啶)和1-oxa-6-氮杂螺[4.5]癸烷((-)-番茄甾醇)核。薯gen皂素,具有两个立体选择性的级联转化,包括改良的呋喃斯坦-26-酸级联环转换过程以打开(-)-薯-皂苷元的E环,以及叠氮化物还原/分子内还原胺化以关闭E-和F- (-)-茄啶和(-)-番茄甾醇的环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号