Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
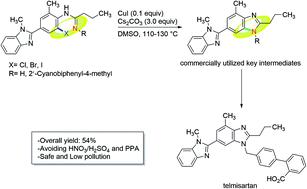
An improved synthesis of telmisartan via the copper-catalyzed cyclization of o-haloarylamidines
RSC Advances ( IF 3.9 ) Pub Date : 2020-4-3 , DOI: 10.1039/d0ra00886a Junchi Zhang 1, 2 , Rui Li 1, 2 , Fuqiang Zhu 3 , Changliang Sun 3 , Jingshan Shen 1, 2
RSC Advances ( IF 3.9 ) Pub Date : 2020-4-3 , DOI: 10.1039/d0ra00886a Junchi Zhang 1, 2 , Rui Li 1, 2 , Fuqiang Zhu 3 , Changliang Sun 3 , Jingshan Shen 1, 2
Affiliation
|
A concise synthetic route was designed for making telmisartan. The key bis-benzimidazole structure was constructed via the copper-catalyzed cyclization of o-haloarylamidines. By adopting this approach, telmisartan was obtained in a 7-step overall yield of 54% starting from commercially available 3-methyl-4-nitrobenzoic acid, and the use of HNO3/H2SO4 for nitration and polyphosphoric acid (PPA) for cyclization in the reported literatures were avoided.
中文翻译:

铜催化邻卤代芳胺环化合成替米沙坦的改进
为制备替米沙坦设计了一条简明的合成路线。关键的双苯并咪唑结构是通过铜催化的邻卤代芳基脒的环化反应构建的。采用这种方法,从市售的 3-甲基-4-硝基苯甲酸开始,使用 HNO 3 /H 2 SO 4进行硝化和多聚磷酸 (PPA) ,经过 7 步总收率 54% 得到替米沙坦避免了报道文献中的环化。
更新日期:2020-04-03
中文翻译:

铜催化邻卤代芳胺环化合成替米沙坦的改进
为制备替米沙坦设计了一条简明的合成路线。关键的双苯并咪唑结构是通过铜催化的邻卤代芳基脒的环化反应构建的。采用这种方法,从市售的 3-甲基-4-硝基苯甲酸开始,使用 HNO 3 /H 2 SO 4进行硝化和多聚磷酸 (PPA) ,经过 7 步总收率 54% 得到替米沙坦避免了报道文献中的环化。










































 京公网安备 11010802027423号
京公网安备 11010802027423号