Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of highly substituted tetrahydroquinolines using ethyl cyanoacetate via aza-Michael–Michael addition
RSC Advances ( IF 3.9 ) Pub Date : 2020-4-3 , DOI: 10.1039/d0ra01264e Arunan Palanimuthu, Chinpiao Chen, Gene-Hsian Lee
RSC Advances ( IF 3.9 ) Pub Date : 2020-4-3 , DOI: 10.1039/d0ra01264e Arunan Palanimuthu, Chinpiao Chen, Gene-Hsian Lee
|
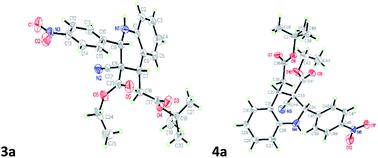
A three-component cascade reaction involving 2-alkenyl aniline, aldehydes, and ethyl cyanoacetate in the presence of DBU to synthesize highly substituted 1,2,3,4-tetrahydroquinolines is reported. The reaction proceeded through the Knoevenagel condensation of ethyl cyanoacetate with aldehydes followed by the aza-Michael–Michael addition with 2-alkenyl anilines to prepare the tetrahydroquinoline scaffolds.
中文翻译:

使用氰乙酸乙酯通过氮杂-迈克尔-迈克尔加成合成高度取代的四氢喹啉
据报道,在 DBU 存在下,涉及 2-烯基苯胺、醛和氰基乙酸乙酯的三组分级联反应合成了高度取代的 1,2,3,4-四氢喹啉。该反应通过氰基乙酸乙酯与醛的 Knoevenagel 缩合进行,然后与 2-烯基苯胺进行氮杂-迈克尔-迈克尔加成,制备四氢喹啉支架。
更新日期:2020-04-03
中文翻译:

使用氰乙酸乙酯通过氮杂-迈克尔-迈克尔加成合成高度取代的四氢喹啉
据报道,在 DBU 存在下,涉及 2-烯基苯胺、醛和氰基乙酸乙酯的三组分级联反应合成了高度取代的 1,2,3,4-四氢喹啉。该反应通过氰基乙酸乙酯与醛的 Knoevenagel 缩合进行,然后与 2-烯基苯胺进行氮杂-迈克尔-迈克尔加成,制备四氢喹啉支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号