PLOS ONE ( IF 2.9 ) Pub Date : 2020-04-02 , DOI: 10.1371/journal.pone.0231050 Marci K Sontag 1, 2 , Joshua I Miller 1, 2 , Sarah McKasson 1, 2 , Ruthanne Sheller 3 , Sari Edelman 3 , Careema Yusuf 3 , Sikha Singh 3 , Deboshree Sarkar 4 , Joseph Bocchini 5 , Joan Scott 4 , Jelili Ojodu 3 , Yvonne Kellar-Guenther 1, 6
|
Background
Newborn screening (NBS) aims to achieve early identification and treatment of affected infants prior to onset of symptoms. The timely completion of each step (i.e., specimen collection, transport, testing, result reporting), is critical for early diagnosis. Goals developed by the Secretary of Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) for NBS timeliness were adopted (time-critical results reported by five days of life, and non-time-critical results reported by day seven), and implemented into a multi-year quality improvement initiative (NewSTEPS 360) aimed to decrease the time to result reporting and intervention.
Methods
The NBS system from specimen collection through reporting of results was assessed (bloodspot specimen collection, specimen shipping, sample testing, and result reporting). Annual data from 25 participating NBS programs were analyzed; the medians (and interquartile range, IQR) of state-specific percent of specimens that met the goal are presented.
Results
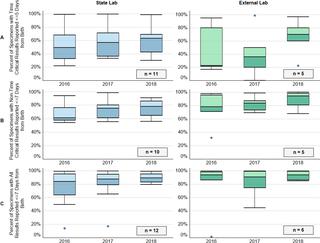
The percent of specimens collected before 48 hours of life increased from 95% (88–97%) in 2016 to 97% (IQR 92–98%) in 2018 for the 25 states, with 20 (80%) of programs collecting more than 90% of the specimens within 48 hours of birth. Approximately 41% (IQR 29–57%) of specimens were transported within one day of collection. Time-critical result reporting in the first five days of life improved from 49% (IQR 26–74%) in 2016 to 64% (42%-71%) in 2018, and for non-time critical results from 64% (IQR 58%-78%) in 2016 to 81% (IQR 68–91%) in 2018. Laboratories open seven days a week in 2018 reported 95% of time-critical results within five days, compared to those open six days (62%), and five days (45%).
Conclusion
NBS programs that participated in NewSTEPs 360 made great strides in improving timeliness; however, ongoing quality improvement efforts are needed in order to ensure all infants receive a timely diagnosis.
中文翻译:

新生儿筛查及时性质量改进举措:国家建议和数据存储库的影响。
背景
新生儿筛查 (NBS) 旨在在症状出现之前早期识别和治疗受影响的婴儿。及时完成每个步骤(即标本采集、运输、检测、结果报告)对于早期诊断至关重要。采用了卫生与公共服务部部长新生儿和儿童遗传性疾病咨询委员会 (ACHDNC) 为 NBS 及时性制定的目标(对时间要求严格的结果按出生后 5 天报告,对时间要求严格的结果按天报告)七),并实施到多年质量改进计划(NewSTEPS 360)中,旨在减少结果报告和干预的时间。
方法
对从标本采集到结果报告的 NBS 系统进行了评估(血斑标本采集、标本运输、样本检测和结果报告)。对 25 个参与的国家统计局项目的年度数据进行了分析;给出了达到目标的特定州样本百分比的中位数(和四分位数范围,IQR)。
结果
对于 25 个州,在生命 48 小时之前收集的样本百分比从 2016 年的 95% (88–97%) 增加到 2018 年的 97% (IQR 92–98%),其中 20 个项目 (80%) 收集了超过90%的标本在出生后48小时内。大约 41% (IQR 29–57%) 的标本在采集后一天内完成运输。生命前五天的时间关键结果报告从 2016 年的 49% (IQR 26–74%) 提高到 2018 年的 64% (42%-71%),非时间关键结果从 64% (IQR 2016 年的 58%-78%)上升到 2018 年的 81%(IQR 68-91%)。2018 年每周开放 7 天的实验室在五天内报告了 95% 的时间关键型结果,而开放六天的实验室(62% )和五天(45%)。
结论
参与 NewSTEPs 360 的 NBS 项目在提高时效性方面取得了长足进步;然而,需要持续不断的质量改进工作,以确保所有婴儿都能得到及时诊断。











































 京公网安备 11010802027423号
京公网安备 11010802027423号