Nature Communications ( IF 16.6 ) Pub Date : 2020-04-03 , DOI: 10.1038/s41467-020-15241-9 Stefan Peissert 1 , Florian Sauer 1 , Daniel B Grabarczyk 1 , Cathy Braun 2, 3, 4, 5 , Gudrun Sander 1 , Arnaud Poterszman 2, 3, 4, 5 , Jean-Marc Egly 2, 3, 4, 5 , Jochen Kuper 1 , Caroline Kisker 1, 6
|
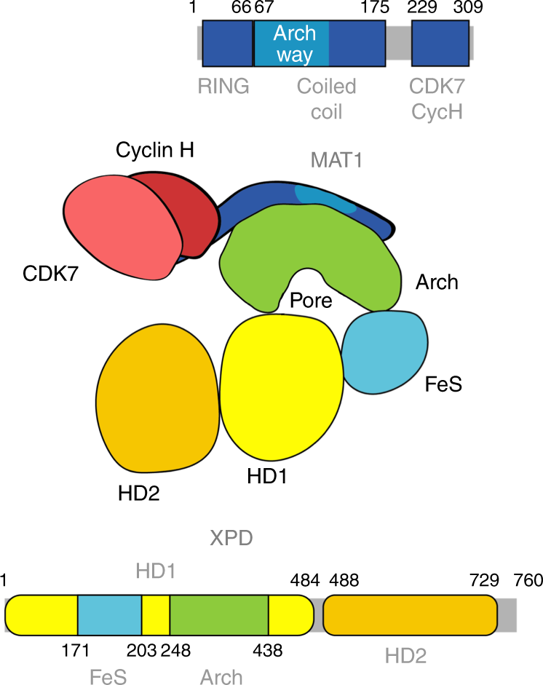
The XPD helicase is a central component of the general transcription factor TFIIH which plays major roles in transcription and nucleotide excision repair (NER). Here we present the high-resolution crystal structure of the Arch domain of XPD with its interaction partner MAT1, a central component of the CDK activating kinase complex. The analysis of the interface led to the identification of amino acid residues that are crucial for the MAT1-XPD interaction. More importantly, mutagenesis of the Arch domain revealed that these residues are essential for the regulation of (i) NER activity by either impairing XPD helicase activity or the interaction of XPD with XPG; (ii) the phosphorylation of the RNA polymerase II and RNA synthesis. Our results reveal how MAT1 shields these functionally important residues thereby providing insights into how XPD is regulated by MAT1 and defining the Arch domain as a major mechanistic player within the XPD scaffold.
中文翻译:

在TFIIH中,XPD的Arch结构域对于转录和DNA修复是机械上必不可少的。
XPD解旋酶是一般转录因子TFIIH的重要组成部分,它在转录和核苷酸切除修复(NER)中起主要作用。在这里,我们介绍XPD的Arch结构域的高分辨率晶体结构及其相互作用伙伴MAT1,后者是CDK激活激酶复合物的重要组成部分。对界面的分析导致了对MAT1-XPD相互作用至关重要的氨基酸残基的鉴定。更重要的是,Arch结构域的诱变表明,这些残基对于调节(i)通过削弱XPD解旋酶活性或XPD与XPG的相互作用的NER活性至关重要。(ii)RNA聚合酶II的磷酸化和RNA合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号