Nature Communications ( IF 14.7 ) Pub Date : 2020-04-03 , DOI: 10.1038/s41467-020-15517-0 Iskander Khusainov 1, 2, 3 , Bulat Fatkhullin 1, 4 , Simone Pellegrino 2, 5 , Aydar Bikmullin 1 , Wen-Ti Liu 6 , Azat Gabdulkhakov 4 , Amr Al Shebel 1 , Alexander Golubev 1, 2 , Denis Zeyer 6 , Natalie Trachtmann 1, 7 , Georg A Sprenger 7 , Shamil Validov 1 , Konstantin Usachev 1 , Gulnara Yusupova 2 , Marat Yusupov 1, 2
|
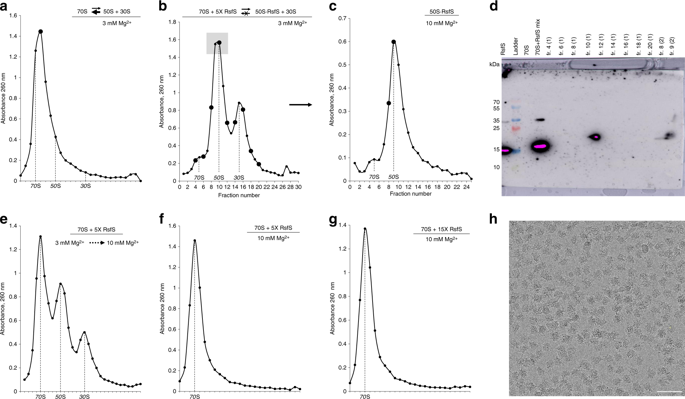
For the sake of energy preservation, bacteria, upon transition to stationary phase, tone down their protein synthesis. This process is favored by the reversible binding of small stress-induced proteins to the ribosome to prevent unnecessary translation. One example is the conserved bacterial ribosome silencing factor (RsfS) that binds to uL14 protein onto the large ribosomal subunit and prevents its association with the small subunit. Here we describe the binding mode of Staphylococcus aureus RsfS to the large ribosomal subunit and present a 3.2 Å resolution cryo-EM reconstruction of the 50S-RsfS complex together with the crystal structure of uL14-RsfS complex solved at 2.3 Å resolution. The understanding of the detailed landscape of RsfS-uL14 interactions within the ribosome shed light on the mechanism of ribosome shutdown in the human pathogen S. aureus and might deliver a novel target for pharmacological drug development and treatment of bacterial infections.
中文翻译:

综合结构生物学方法揭示金黄色葡萄球菌RsfS核糖体关闭的机制。
为了节省能量,细菌在过渡到固定相时会降低其蛋白质合成。小应力诱导的蛋白质与核糖体的可逆结合有利于此过程,以防止不必要的翻译。一个例子是保守的细菌核糖体沉默因子(RsfS),它与uL14蛋白结合到大的核糖体亚基上并阻止其与小亚基结合。在这里我们描述金黄色葡萄球菌的结合模式RsfS到大的核糖体亚基,并以3.2Å分辨率解析了50S-RsfS复合物的3.2Å分辨率冷冻-EM重建以及uL14-RsfS复杂的晶体结构。对核糖体内部RsfS-uL14相互作用的详细情况的了解为人类病原体金黄色葡萄球菌核糖体关闭的机制提供了线索,并可能为药理药物开发和细菌感染的治疗提供新目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号