当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ki-67 response-guided preoperative chemotherapy for HER2-positive breast cancer: results of a randomised Phase 2 study.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-04-02 , DOI: 10.1038/s41416-020-0815-9 Hirofumi Mukai 1 , Takeshi Yamaguchi 2 , Masato Takahashi 3 , Yasuo Hozumi 4 , Tomomi Fujisawa 5 , Shozo Ohsumi 6 , Hiromitsu Akabane 7 , Reiki Nishimura 8 , Tsutomu Takashima 9 , Youngjin Park 10 , Yasuaki Sagara 11 , Tatsuya Toyama 12 , Shigeru Imoto 13 , Toshiro Mizuno 14 , Satoshi Yamashita 15 , Satoshi Fujii 16 , Yukari Uemura 17
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-04-02 , DOI: 10.1038/s41416-020-0815-9 Hirofumi Mukai 1 , Takeshi Yamaguchi 2 , Masato Takahashi 3 , Yasuo Hozumi 4 , Tomomi Fujisawa 5 , Shozo Ohsumi 6 , Hiromitsu Akabane 7 , Reiki Nishimura 8 , Tsutomu Takashima 9 , Youngjin Park 10 , Yasuaki Sagara 11 , Tatsuya Toyama 12 , Shigeru Imoto 13 , Toshiro Mizuno 14 , Satoshi Yamashita 15 , Satoshi Fujii 16 , Yukari Uemura 17
Affiliation
|
BACKGROUND
The effectiveness of a therapeutic strategy that switches chemotherapy, based on Ki-67 tumour expression after initial therapy, relative to that of standard chemotherapy, has not been evaluated.
METHODS
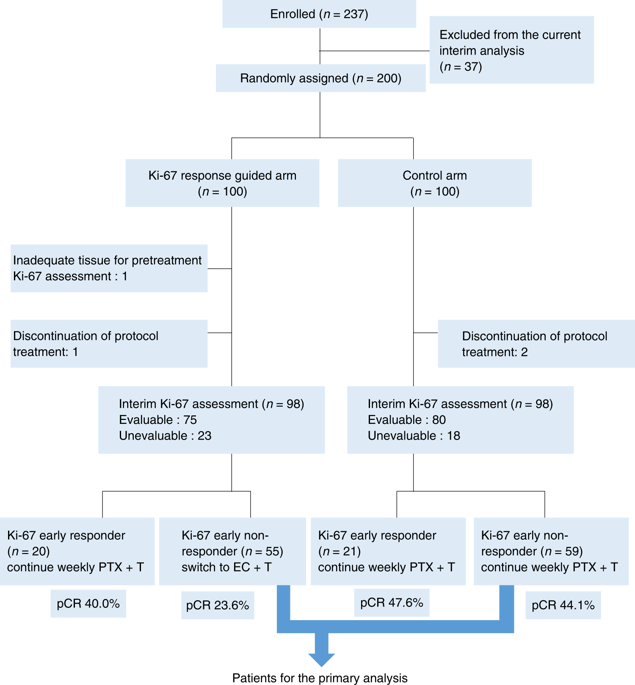
Patients were randomly assigned to the control arm or the Ki-67 response-guided arm (Ki-67 arm). Primary tumour biopsies were obtained before treatment, and after three once-weekly doses of paclitaxel and trastuzumab to assess the interim Ki-67 index. In the control arm, paclitaxel and trastuzumab were continued for a total of 12 doses, regardless of the interim Ki-67 index. In the Ki-67 arm, subsequent treatment was based on the interim Ki-67 index. Ki-67 early responder is defined as the absolute Ki-67 value that was <10%, and the percentage of Ki-67-positive tumour cells was reduced by >30% compared with before treatment. Early Ki-67 responders continued to receive the same treatment, while early Ki-67 non-responders were switched to epirubicin plus cyclophosphamide. The primary endpoint was the pathological complete response (pCR) rate.
RESULTS
A total of 237 patients were randomised. There was almost linear correlation between the Ki-67 reduction rate at interim assessment and the pCR rate. The pCR rate in Ki-67 early non-responders in the Ki-67 arm was inferior to that in the control arm (44.1%; 31.4-56.7; P = 0.025).
CONCLUSIONS
The standard chemotherapy protocol remains as the recommended strategy for patients with HER2-positive breast cancer.
CLINICAL TRIAL REGISTRATION
Clinical Trial Registration: UMIN-CTR as UMIN000007074.
中文翻译:

Ki-67反应指导的HER2阳性乳腺癌术前化疗:一项随机2期研究的结果。
背景技术相对于标准化学疗法,尚未评估基于初始治疗后基于Ki-67肿瘤表达的切换化学疗法的治疗策略的有效性。方法将患者随机分为对照组或Ki-67反应指导组(Ki-67组)。在治疗前和每周三次紫杉醇和曲妥珠单抗给药后获得原发性肿瘤活检,以评估中期Ki-67指数。在对照组中,无论中期Ki-67指数如何,紫杉醇和曲妥珠单抗共持续服用12剂。在Ki-67组,后续治疗基于中期Ki-67指数。Ki-67早期反应者定义为绝对Ki-67值<10%,并且与治疗前相比,Ki-67阳性肿瘤细胞的百分比降低> 30%。早期的Ki-67反应者继续接受相同的治疗,而早期的Ki-67无反应者改用表柔比星加环磷酰胺。主要终点是病理完全缓解率(pCR)。结果共有237例患者被随机分组。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。而早期的Ki-67无反应者改用表柔比星加环磷酰胺。主要终点是病理完全缓解率(pCR)。结果共有237例患者被随机分组。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。而早期的Ki-67无反应者转为表柔比星加环磷酰胺。主要终点是病理完全缓解率(pCR)。结果共有237例患者被随机分组。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。
更新日期:2020-04-24
中文翻译:

Ki-67反应指导的HER2阳性乳腺癌术前化疗:一项随机2期研究的结果。
背景技术相对于标准化学疗法,尚未评估基于初始治疗后基于Ki-67肿瘤表达的切换化学疗法的治疗策略的有效性。方法将患者随机分为对照组或Ki-67反应指导组(Ki-67组)。在治疗前和每周三次紫杉醇和曲妥珠单抗给药后获得原发性肿瘤活检,以评估中期Ki-67指数。在对照组中,无论中期Ki-67指数如何,紫杉醇和曲妥珠单抗共持续服用12剂。在Ki-67组,后续治疗基于中期Ki-67指数。Ki-67早期反应者定义为绝对Ki-67值<10%,并且与治疗前相比,Ki-67阳性肿瘤细胞的百分比降低> 30%。早期的Ki-67反应者继续接受相同的治疗,而早期的Ki-67无反应者改用表柔比星加环磷酰胺。主要终点是病理完全缓解率(pCR)。结果共有237例患者被随机分组。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。而早期的Ki-67无反应者改用表柔比星加环磷酰胺。主要终点是病理完全缓解率(pCR)。结果共有237例患者被随机分组。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。而早期的Ki-67无反应者转为表柔比星加环磷酰胺。主要终点是病理完全缓解率(pCR)。结果共有237例患者被随机分组。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。中期评估中Ki-67降低率与pCR率之间几乎呈线性相关。Ki-67组的Ki-67早期无反应者的pCR率低于对照组(44.1%; 31.4-56.7; P = 0.025)。结论标准的化疗方案仍然是HER2阳性乳腺癌患者的推荐策略。临床试验注册临床试验注册:UMIN-CTR,名称为UMIN000007074。











































 京公网安备 11010802027423号
京公网安备 11010802027423号