JAMA Oncology ( IF 22.5 ) Pub Date : 2020-07-01 , DOI: 10.1001/jamaoncol.2020.0249 Jayant S Vaidya 1 , Max Bulsara 1, 2 , Christobel Saunders 3 , Henrik Flyger 4 , Jeffrey S Tobias 5 , Tammy Corica 6 , Samuele Massarut 7 , Frederik Wenz 8 , Steffi Pigorsch 9 , Michael Alvarado 10 , Michael Douek 11 , Wolfgang Eiermann 9 , Chris Brew-Graves 1 , Norman Williams 1 , Ingrid Potyka 1 , Nicholas Roberts 1 , Marcelle Bernstein 12 , Douglas Brown 13 , Elena Sperk 8 , Siobhan Laws 14 , Marc Sütterlin 15 , Steinar Lundgren 16, 17 , Dennis Holmes 18 , Lorenzo Vinante 19 , Fernando Bozza 20 , Montserrat Pazos 21 , Magali Le Blanc-Onfroy 22 , Günther Gruber 23 , Wojciech Polkowski 24 , Konstantin J Dedes 25 , Marcus Niewald 26 , Jens Blohmer 27 , David McCready 28 , Richard Hoefer 29 , Pond Kelemen 30 , Gloria Petralia 31 , Mary Falzon 32 , Michael Baum 1 , David Joseph 6
|
Importance Conventional adjuvant radiotherapy for breast cancer given daily for several weeks is onerous and expensive. Some patients may be obliged to choose a mastectomy instead, and some may forgo radiotherapy altogether. We proposed a clinical trial to test whether radiotherapy could be safely limited to the tumor bed.
Objective To determine whether delayed second-procedure targeted intraoperative radiotherapy (TARGIT-IORT) is noninferior to whole-breast external beam radiotherapy (EBRT) in terms of local control.
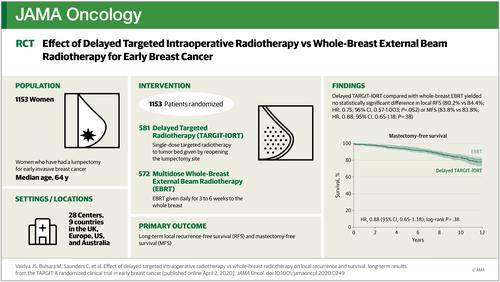
Design, Setting, and Participants In this prospective, randomized (1:1 ratio) noninferiority trial, 1153 patients aged 45 years or older with invasive ductal breast carcinoma smaller than 3.5 cm treated with breast conservation were enrolled from 28 centers in 9 countries. Data were locked in on July 3, 2019.
Interventions The TARGIT-A trial was started in March 2000; patients were randomized after needle biopsy to receive TARGIT-IORT immediately after lumpectomy under the same anesthetic vs EBRT and results have been shown to be noninferior. A parallel study, described in this article, was initiated in 2004; patients who had their cancer excised were randomly allocated using separate randomization tables to receive EBRT or delayed TARGIT-IORT given as a second procedure by reopening the lumpectomy wound.
Main Outcomes and Measures A noninferiority margin for local recurrence rate of 2.5% at 5 years, and long-term survival outcomes.
Results Overall, 581 women (mean [SD] age, 63 [7] years) were randomized to delayed TARGIT-IORT and 572 patients (mean [SD] age, 63 [8] years) were randomized to EBRT. Sixty patients (5%) had tumors larger than 2 cm, or had positive nodes and only 32 (2.7%) were younger than 50 years. Delayed TARGIT-IORT was not noninferior to EBRT. The local recurrence rates at 5-year complete follow-up were: delayed TARGIT-IORT vs EBRT (23/581 [3.96%] vs 6/572 [1.05%], respectively; difference, 2.91%; upper 90% CI, 4.4%). With long-term follow-up (median [IQR], 9.0 [7.5-10.5] years), there was no statistically significant difference in local recurrence-free survival (HR, 0.75; 95% CI, 0.57-1.003; P = .052), mastectomy-free survival (HR, 0.88; 95% CI, 0.65-1.18; P = .38), distant disease-free survival (HR, 1.00; 95% CI, 0.72-1.39; P = .98), or overall survival (HR, 0.96; 95% CI, 0.68-1.35; P = .80).
Conclusions and Relevance These long-term data show that despite an increase in the number of local recurrences with delayed TARGIT-IORT, there was no statistically significant decrease in mastectomy-free survival, distant disease-free survival, or overall survival.
Trial Registration ISRCTN34086741, ClinicalTrials.gov Identifier: NCT00983684
中文翻译:

延迟靶向术中放疗与全乳放疗对局部复发和生存的影响:TARGIT-A 早期乳腺癌随机临床试验的长期结果。
重要性 连续数周每天对乳腺癌进行常规辅助放疗既繁重又昂贵。有些患者可能不得不选择乳房切除术,而有些患者可能会完全放弃放射治疗。我们提出了一项临床试验,以测试放射治疗是否可以安全地限制在肿瘤床内。
目的 确定延迟二期手术靶向术中放疗(TARGIT-IORT)在局部控制方面是否不劣于全乳外照射放疗(EBRT)。
设计、设置和参与者 在这项前瞻性、随机(1:1 比率)非劣效性试验中,来自 9 个国家的 28 个中心的 1153 名年龄在 45 岁或以上且接受保乳治疗的直径小于 3.5 cm 的浸润性导管乳腺癌患者入组。数据于 2019 年 7 月 3 日锁定。
干预 TARGIT-A 试验于 2000 年 3 月开始;患者在穿刺活检后被随机分配接受 TARGIT-IORT 在肿瘤切除术后立即接受相同的麻醉与 EBRT 相比,结果已被证明是非劣效的。本文描述的一项平行研究始于 2004 年;切除癌症的患者使用单独的随机表随机分配接受 EBRT 或延迟 TARGIT-IORT 作为第二次手术,通过重新打开肿块切除术伤口。
主要结果和措施 5 年时局部复发率为 2.5% 和长期生存结果的非劣效性界值。
结果 总体而言,581 名女性(平均 [SD] 年龄,63 [7] 岁)被随机分配至延迟 TARGIT-IORT,572 名患者(平均 [SD] 年龄,63 [8] 岁)被随机分配至 EBRT。60 名患者 (5%) 的肿瘤大于 2 cm,或淋巴结阳性,只有 32 名 (2.7%) 的患者年龄小于 50 岁。延迟 TARGIT-IORT 并不劣于 EBRT。5 年完整随访时的局部复发率为:延迟 TARGIT-IORT 与 EBRT(分别为 23/581 [3.96%] vs 6/572 [1.05%];差异,2.91%;上 90% CI,4.4 %)。长期随访(中位 [IQR],9.0 [7.5-10.5] 年),局部无复发生存期无统计学差异(HR,0.75;95% CI,0.57-1.003;P = . 052),无乳房切除术生存率(HR,0.88;95% CI,0.65-1.18;P = .38)、远处无病生存期 (HR, 1.00; 95% CI, 0.72-1.39; P = .98) 或总生存期 (HR, 0.96; 95% CI, 0.68-1.35; P = .80) .
结论和相关性 这些长期数据表明,尽管延迟 TARGIT-IORT 的局部复发数量有所增加,但无乳房切除术生存率、无远处无病生存率或总生存率没有统计学意义的下降。
试验注册 ISRCTN34086741,ClinicalTrials.gov 标识符:NCT00983684











































 京公网安备 11010802027423号
京公网安备 11010802027423号