当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of disparlure and monachalure enantiomers from 2,3-butanediacetals
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-04-03 , DOI: 10.3762/bjoc.16.57 Adam Drop , Hubert Wojtasek , Bożena Frąckowiak-Wojtasek
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-04-03 , DOI: 10.3762/bjoc.16.57 Adam Drop , Hubert Wojtasek , Bożena Frąckowiak-Wojtasek
|
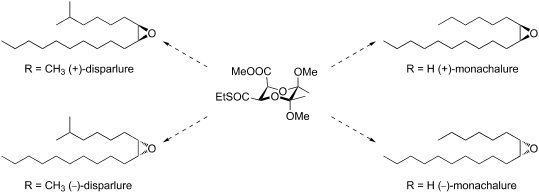
2,3-Butanediacetal derivatives were used for the stereoselective synthesis of unsymmetrically substituted cis-epoxides. The procedure was applied for the preparation of both enantiomers of disparlure and monachalure, the components of the sex pheromones of the gypsy moth (Lymantria dispar) and the nun moth (Lymantria monacha) using methyl (2S,3R,5R,6R)-3-ethylsulfanylcarbonyl-5,6-dimethoxy-5,6-dimethyl-1,4-dioxane-2-carboxylate as the starting material.
中文翻译:

由2,3-丁二缩醛合成异戊二烯和莫纳丘利尔对映体
2,3-丁二缩醛衍生物用于不对称取代的顺式环氧化物的立体选择性合成。该方法适用于使用甲基(2 S,3 R,5 R,6 )制备Disparlure和Monachalure的对映异构体,吉普赛蛾(Lymantria dispar)和尼姑蛾(Lymantria monacha)的性信息素的成分。R)-3-乙基硫烷基羰基-5,6-二甲氧基-5,6-二甲基-1,4-二恶烷-2-羧酸酯为起始原料。
更新日期:2020-04-03
中文翻译:

由2,3-丁二缩醛合成异戊二烯和莫纳丘利尔对映体
2,3-丁二缩醛衍生物用于不对称取代的顺式环氧化物的立体选择性合成。该方法适用于使用甲基(2 S,3 R,5 R,6 )制备Disparlure和Monachalure的对映异构体,吉普赛蛾(Lymantria dispar)和尼姑蛾(Lymantria monacha)的性信息素的成分。R)-3-乙基硫烷基羰基-5,6-二甲氧基-5,6-二甲基-1,4-二恶烷-2-羧酸酯为起始原料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号