当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An assessment study of known pyrazolopyrimidines: Chemical methodology and cellular activity.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.bioorg.2020.103801 R Nishanth Rao 1 , Kaushik Chanda 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.bioorg.2020.103801 R Nishanth Rao 1 , Kaushik Chanda 1
Affiliation
|
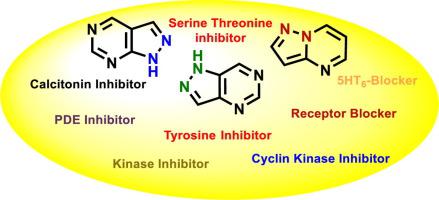
Heterocyclic compounds with nitrogen atom play a key role in the normal life cycle of a cell. Pyrazolopyrimidine is a privileged class of nitrogen containing fused heterocyclic compound contributing to a major portion of all lead molecules in medicinal chemistry. The thumbprint of pyrazolopyrimidine as a pharmacophore is always noticeable due to its analogy with the adenine base in DNA. Pyrazolopyrimidines are divided into five types [I, II, III, IV, V] based on the mechanism of action on the specific target conferring a wide scope of research which has accelerated the interest of researchers to investigate its biological profile. In 1956, the anti-cancer activity of pyrazolopyrimidine was evaluated for the first time with appreciable results. Since then, medicinal chemists centered their work on various methods of synthesis and evaluating the biological profile of pyrazolopyrimidine isomers. This report consists of novel methodologies followed to synthesize pyrazolopyrimidine isomers along with a note on their biological significance. To the best of our knowledge, this review article will be first of its kind to encompass different synthetic procedures along with anti-cancer, kinase inhibition, phosphodiesterase inhibition and receptor blocking activity of pyrazolopyrimidine moieties. IC50 values of potent compounds are added wherever necessary to understand the suitability of pyrazolopyrimidine skeletons for a specific biological activity.
中文翻译:

已知吡唑并嘧啶的评估研究:化学方法和细胞活性。
具有氮原子的杂环化合物在细胞的正常生命周期中起着关键作用。吡唑并嘧啶是一类特权的含氮稠合杂环化合物,在药物化学中占所有铅分子的主要部分。吡唑并嘧啶作为药效基团的指纹始终很引人注目,因为它与DNA中的腺嘌呤碱基类似。基于对特定靶标的作用机理,吡唑并嘧啶分为五种类型[I,II,III,IV,V],这赋予了广泛的研究范围,这加速了研究人员研究其生物学特性的兴趣。1956年,首次评估了吡唑并嘧啶的抗癌活性,并得出了可观的结果。自那时候起,药物化学家的工作集中在各种合成方法上,并评估了吡唑并嘧啶异构体的生物学特性。该报告由随后合成吡唑并嘧啶异构体的新方法以及有关其生物学意义的注释组成。就我们所知,这篇综述文章将是同类文章中的第一篇,涵盖了吡唑并嘧啶部分的抗癌,激酶抑制,磷酸二酯酶抑制和受体阻断活性以及不同的合成方法。如有必要,可添加有效化合物的IC50值,以了解吡唑并嘧啶骨架对特定生物活性的适用性。该报告由随后合成吡唑并嘧啶异构体的新方法以及有关其生物学意义的注释组成。就我们所知,这篇综述文章将是同类文章中的第一篇,涵盖了吡唑并嘧啶部分的抗癌,激酶抑制,磷酸二酯酶抑制和受体阻断活性以及不同的合成方法。如有必要,可添加有效化合物的IC50值,以了解吡唑并嘧啶骨架对特定生物活性的适用性。该报告由随后合成吡唑并嘧啶异构体的新方法以及有关其生物学意义的注释组成。就我们所知,这篇综述文章将是同类文章中的第一篇,涵盖了吡唑并嘧啶部分的抗癌,激酶抑制,磷酸二酯酶抑制和受体阻断活性以及不同的合成方法。如有必要,可添加有效化合物的IC50值,以了解吡唑并嘧啶骨架对特定生物活性的适用性。
更新日期:2020-04-20
中文翻译:

已知吡唑并嘧啶的评估研究:化学方法和细胞活性。
具有氮原子的杂环化合物在细胞的正常生命周期中起着关键作用。吡唑并嘧啶是一类特权的含氮稠合杂环化合物,在药物化学中占所有铅分子的主要部分。吡唑并嘧啶作为药效基团的指纹始终很引人注目,因为它与DNA中的腺嘌呤碱基类似。基于对特定靶标的作用机理,吡唑并嘧啶分为五种类型[I,II,III,IV,V],这赋予了广泛的研究范围,这加速了研究人员研究其生物学特性的兴趣。1956年,首次评估了吡唑并嘧啶的抗癌活性,并得出了可观的结果。自那时候起,药物化学家的工作集中在各种合成方法上,并评估了吡唑并嘧啶异构体的生物学特性。该报告由随后合成吡唑并嘧啶异构体的新方法以及有关其生物学意义的注释组成。就我们所知,这篇综述文章将是同类文章中的第一篇,涵盖了吡唑并嘧啶部分的抗癌,激酶抑制,磷酸二酯酶抑制和受体阻断活性以及不同的合成方法。如有必要,可添加有效化合物的IC50值,以了解吡唑并嘧啶骨架对特定生物活性的适用性。该报告由随后合成吡唑并嘧啶异构体的新方法以及有关其生物学意义的注释组成。就我们所知,这篇综述文章将是同类文章中的第一篇,涵盖了吡唑并嘧啶部分的抗癌,激酶抑制,磷酸二酯酶抑制和受体阻断活性以及不同的合成方法。如有必要,可添加有效化合物的IC50值,以了解吡唑并嘧啶骨架对特定生物活性的适用性。该报告由随后合成吡唑并嘧啶异构体的新方法以及有关其生物学意义的注释组成。就我们所知,这篇综述文章将是同类文章中的第一篇,涵盖了吡唑并嘧啶部分的抗癌,激酶抑制,磷酸二酯酶抑制和受体阻断活性以及不同的合成方法。如有必要,可添加有效化合物的IC50值,以了解吡唑并嘧啶骨架对特定生物活性的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号