当前位置:
X-MOL 学术
›
Curr. Opin. Biotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroporation as a method of choice to generate genetically modified dendritic cell cancer vaccines.
Current Opinion in Biotechnology ( IF 7.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.copbio.2020.02.009 Rita Ahmed 1 , Naya Sayegh 1 , Michele Graciotti 1 , Lana E Kandalaft 1
Current Opinion in Biotechnology ( IF 7.1 ) Pub Date : 2020-03-30 , DOI: 10.1016/j.copbio.2020.02.009 Rita Ahmed 1 , Naya Sayegh 1 , Michele Graciotti 1 , Lana E Kandalaft 1
Affiliation
|
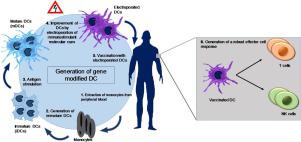
In the last few decades, immunotherapy has emerged as an alternative therapeutic approach to treat cancer. Immunotherapy offers a plethora of different treatment possibilities. Among these, dendritic cell (DC)-based cancer vaccines constitute one of the most promising and valuable therapeutic options. DC-vaccines have been introduced into the clinics more than 15 years ago, and preclinical studies showed their general safety and low toxic effects on patients. However, their treatment efficacy is still rather limited, demanding for novel avenues to improve vaccine efficacy. One way to potentially achieve this is to focus on improving the DC-T cell interaction to further increase T cell priming and downstream activity. A successful DC-T cell interaction requires three different signals (Figure 1): (1) Major Histocompatibility Complex (MHC) and antigen complex interaction with T cell receptor (TCR) (2) interaction between co-stimulatory molecules and their cognate ligands at the cell surface and (3) secretion of cytokines to polarize the immune response toward a Type 1 helper (Th1) phenotype. In recent years, many studies attempted to improve the DC-T cell interaction and overall cancer vaccine therapeutic outcomes by increasing the expression of mediators of signal 1, 2 and/or 3, through genetic modifications of DCs. Transfection of genes of interest can be achieved through many different methods such as passive pulsing, lipofection, viral transfection, or electroporation (EP). However, EP is currently emerging as the method of choice thanks to its safety, versatility, and relatively easy clinical translation. In this review we will highlight the potential benefits of EP over other transfection methods as well as giving an overview of the available studies employing EP to gene-modify DCs in cancer vaccines. Crucial aspects such as safety, feasibility, and gene(s) of choice will be also discussed, together with future perspectives and opportunities for DC genetic engineering.
中文翻译:

电穿孔是产生基因修饰的树突状细胞癌疫苗的一种选择方法。
在过去的几十年中,免疫疗法已成为治疗癌症的另一种治疗方法。免疫疗法提供了多种不同的治疗可能性。其中,基于树突细胞(DC)的癌症疫苗是最有前途和最有价值的治疗选择之一。DC疫苗已于15年前引入临床,临床前研究表明它们具有一般安全性,对患者的毒副作用低。然而,它们的治疗功效仍然相当有限,需要新颖的途径来提高疫苗功效。潜在实现此目的的一种方法是集中于改善DC-T细胞相互作用,以进一步增加T细胞启动和下游活性。成功的DC-T细胞相互作用需要三种不同的信号(图1):(1)主要组织相容性复合物(MHC)和抗原复合物与T细胞受体(TCR)的相互作用(2)共刺激分子与其同源配体在细胞表面之间的相互作用,以及(3)细胞因子的分泌,使免疫应答对1型辅助(Th1)表型。近年来,许多研究试图通过DC的遗传修饰来增加信号1、2和/或3的介体表达,从而改善DC-T细胞的相互作用和整体癌症疫苗的治疗效果。目的基因的转染可以通过许多不同的方法来实现,例如被动脉冲,脂质转染,病毒转染或电穿孔(EP)。但是,由于其安全性,多功能性和相对容易的临床翻译,EP目前正成为首选方法。在这篇综述中,我们将重点介绍EP相对于其他转染方法的潜在优势,并概述使用EP对癌症疫苗中的DC进行基因修饰的现有研究。还将讨论至关重要的方面,例如安全性,可行性和选择的基因,以及DC基因工程的未来前景和机会。
更新日期:2020-04-20
中文翻译:

电穿孔是产生基因修饰的树突状细胞癌疫苗的一种选择方法。
在过去的几十年中,免疫疗法已成为治疗癌症的另一种治疗方法。免疫疗法提供了多种不同的治疗可能性。其中,基于树突细胞(DC)的癌症疫苗是最有前途和最有价值的治疗选择之一。DC疫苗已于15年前引入临床,临床前研究表明它们具有一般安全性,对患者的毒副作用低。然而,它们的治疗功效仍然相当有限,需要新颖的途径来提高疫苗功效。潜在实现此目的的一种方法是集中于改善DC-T细胞相互作用,以进一步增加T细胞启动和下游活性。成功的DC-T细胞相互作用需要三种不同的信号(图1):(1)主要组织相容性复合物(MHC)和抗原复合物与T细胞受体(TCR)的相互作用(2)共刺激分子与其同源配体在细胞表面之间的相互作用,以及(3)细胞因子的分泌,使免疫应答对1型辅助(Th1)表型。近年来,许多研究试图通过DC的遗传修饰来增加信号1、2和/或3的介体表达,从而改善DC-T细胞的相互作用和整体癌症疫苗的治疗效果。目的基因的转染可以通过许多不同的方法来实现,例如被动脉冲,脂质转染,病毒转染或电穿孔(EP)。但是,由于其安全性,多功能性和相对容易的临床翻译,EP目前正成为首选方法。在这篇综述中,我们将重点介绍EP相对于其他转染方法的潜在优势,并概述使用EP对癌症疫苗中的DC进行基因修饰的现有研究。还将讨论至关重要的方面,例如安全性,可行性和选择的基因,以及DC基因工程的未来前景和机会。







































 京公网安备 11010802027423号
京公网安备 11010802027423号