当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient removal of p-arsanilic acid with Fe(II)/peroxydisulfate under near-neutral conditions.
Water Research ( IF 11.4 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.watres.2020.115752 Panxin Wang 1 , Xu He 1 , Wei Zhang 2 , Jun Ma 1 , Jin Jiang 3 , Zhuangsong Huang 1 , Haijun Cheng 1 , Suyan Pang 4 , Yang Zhou 3 , Xuedong Zhai 1
Water Research ( IF 11.4 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.watres.2020.115752 Panxin Wang 1 , Xu He 1 , Wei Zhang 2 , Jun Ma 1 , Jin Jiang 3 , Zhuangsong Huang 1 , Haijun Cheng 1 , Suyan Pang 4 , Yang Zhou 3 , Xuedong Zhai 1
Affiliation
|
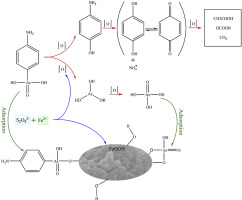
As a common animal feed additive, p-arsanilic acid (p-AsA) is thought to be excreted with little uptake and unchanged chemical structure, threatening the environment by potentially releasing more toxic inorganic arsenic. We herein investigated the removal of arsenic by in situ formed ferric (oxyhydr)oxides with the promotion of p-AsA degradation in Fe(II)/peroxydisulfate (PDS) system. Results showed that under acid conditions, p-AsA degraded very quickly and over 99% of p-AsA (5 μM) was degraded within 10 min at the optimal dosage of Fe(II) (100 μM) and PDS (150 μM) at pH 3, while less than 66.4% of arsenic was removed at pH 3-5. Higher pH (3-7) would inhibit the degradation of p-AsA but promote the arsenic removal. At pH 6-7, over 98.5% of total arsenic was removed, while the degradation efficiency of p-AsA was lower than 52.4%. HPLC-ICP-MS results indicated that the arsenic group was cleaved from p-AsA in the form of As(III) and then rapidly oxidized to As(V). FTIR and XPS analysis indicated that both As(V) products and residual p-AsA were bonded to ferric (oxyhydr)oxides via hydroxyl groups. Common cations (e.g., Na+, Ca2+, Mg2+) and anions such as Cl-, SO42-, CO32- had no significant influence on arsenic removal, while SiO32-, PO43- and HA inhibited the removal of total arsenic, mainly by affecting the zeta potential of iron particles. In summary, the Fe(II)/PDS process, as an efficient method for partial oxidation and simultaneous adsorption of p-AsA under near-neutral conditions, is expected to control the potential environmental risks of p-AsA.
中文翻译:

在接近中性的条件下,用Fe(II)/过二硫酸盐高效去除对砷酸。
作为常见的动物饲料添加剂,对砷酸(p-AsA)被认为以很少的摄入量和不变的化学结构排泄,通过潜在地释放更多有毒的无机砷来威胁环境。我们在本文中研究了在Fe(II)/过二硫酸盐(PDS)系统中促进原位形成的三氧化二铁去除砷并促进p-AsA降解的现象。结果表明,在酸性条件下,在最佳剂量的Fe(II)(100μM)和PDS(150μM)的情况下,在10分钟内p-AsA降解非常快,超过99%的p-AsA(5μM)被降解。 pH为3时,在pH 3-5时除去的砷少于66.4%。pH值较高(3-7)将抑制p-AsA的降解,但促进砷的去除。在pH 6-7时,去除了超过98.5%的总砷,而p-AsA的降解效率低于52.4%。HPLC-ICP-MS结果表明,砷基团以对位AsA(III)的形式从p-AsA上裂解下来,然后迅速氧化为As(V)。FTIR和XPS分析表明,As(V)产物和残留的p-AsA均通过羟基与三价铁(羟基氧化物)结合。常见的阳离子(例如Na +,Ca2 +,Mg2 +)和诸如Cl-,SO42-,CO32-等阴离子对砷的去除没有显着影响,而SiO32-,PO43-和HA则主要通过影响砷的去除而抑制了总砷的去除。铁粒子的ζ电势。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。FTIR和XPS分析表明,As(V)产物和残留的p-AsA均通过羟基与三价铁(羟基氧化物)结合。常见的阳离子(例如Na +,Ca2 +,Mg2 +)和诸如Cl-,SO42-,CO32-等阴离子对砷的去除没有显着影响,而SiO32-,PO43-和HA则主要通过影响砷的去除而抑制了总砷的去除。铁粒子的ζ电势。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。FTIR和XPS分析表明,As(V)产物和残留的p-AsA均通过羟基与三价铁(羟基氧化物)结合。常见的阳离子(例如Na +,Ca2 +,Mg2 +)和诸如Cl-,SO42-,CO32-等阴离子对砷的去除没有显着影响,而SiO32-,PO43-和HA则主要通过影响砷的去除而抑制了总砷的去除。铁粒子的ζ电势。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。而SiO32-,PO43-和HA抑制了总砷的去除,主要是通过影响铁颗粒的ζ电位。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。而SiO32-,PO43-和HA抑制了总砷的去除,主要是通过影响铁颗粒的ζ电位。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。
更新日期:2020-04-03
中文翻译:

在接近中性的条件下,用Fe(II)/过二硫酸盐高效去除对砷酸。
作为常见的动物饲料添加剂,对砷酸(p-AsA)被认为以很少的摄入量和不变的化学结构排泄,通过潜在地释放更多有毒的无机砷来威胁环境。我们在本文中研究了在Fe(II)/过二硫酸盐(PDS)系统中促进原位形成的三氧化二铁去除砷并促进p-AsA降解的现象。结果表明,在酸性条件下,在最佳剂量的Fe(II)(100μM)和PDS(150μM)的情况下,在10分钟内p-AsA降解非常快,超过99%的p-AsA(5μM)被降解。 pH为3时,在pH 3-5时除去的砷少于66.4%。pH值较高(3-7)将抑制p-AsA的降解,但促进砷的去除。在pH 6-7时,去除了超过98.5%的总砷,而p-AsA的降解效率低于52.4%。HPLC-ICP-MS结果表明,砷基团以对位AsA(III)的形式从p-AsA上裂解下来,然后迅速氧化为As(V)。FTIR和XPS分析表明,As(V)产物和残留的p-AsA均通过羟基与三价铁(羟基氧化物)结合。常见的阳离子(例如Na +,Ca2 +,Mg2 +)和诸如Cl-,SO42-,CO32-等阴离子对砷的去除没有显着影响,而SiO32-,PO43-和HA则主要通过影响砷的去除而抑制了总砷的去除。铁粒子的ζ电势。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。FTIR和XPS分析表明,As(V)产物和残留的p-AsA均通过羟基与三价铁(羟基氧化物)结合。常见的阳离子(例如Na +,Ca2 +,Mg2 +)和诸如Cl-,SO42-,CO32-等阴离子对砷的去除没有显着影响,而SiO32-,PO43-和HA则主要通过影响砷的去除而抑制了总砷的去除。铁粒子的ζ电势。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。FTIR和XPS分析表明,As(V)产物和残留的p-AsA均通过羟基与三价铁(羟基氧化物)结合。常见的阳离子(例如Na +,Ca2 +,Mg2 +)和诸如Cl-,SO42-,CO32-等阴离子对砷的去除没有显着影响,而SiO32-,PO43-和HA则主要通过影响砷的去除而抑制了总砷的去除。铁粒子的ζ电势。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。而SiO32-,PO43-和HA抑制了总砷的去除,主要是通过影响铁颗粒的ζ电位。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。而SiO32-,PO43-和HA抑制了总砷的去除,主要是通过影响铁颗粒的ζ电位。总之,Fe(II)/ PDS工艺作为一种在近中性条件下部分氧化和同时吸附p-AsA的有效方法,有望控制p-AsA的潜在环境风险。









































 京公网安备 11010802027423号
京公网安备 11010802027423号