当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Poziotinib and bovine serum albumin binding characterization and influence of quercetin, rutin, naringenin and sinapic acid on their binding interaction.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.saa.2020.118335 Seema Zargar 1 , Salman Alamery 1 , Ahmed H Bakheit 2 , Tanveer A Wani 3
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.saa.2020.118335 Seema Zargar 1 , Salman Alamery 1 , Ahmed H Bakheit 2 , Tanveer A Wani 3
Affiliation
|
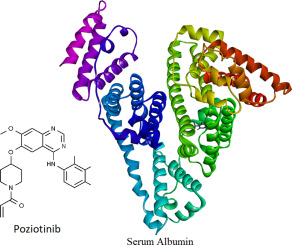
Serum albumin is the major transporter protein present in systemic circulation and the ability to transport ligands can be influenced in presence of other ligands. This interaction can influence the pharmacodynamic and pharmacokinetic property of certain ligands. Spectroscopic and molecular docking studies were conducted to understand the poziotinib binding interaction to bovine serum albumin (BSA). Further, influence of different flavonoids (quercetin, rutin, naringenin and sinapic acid) on displacing poziotinib from BSA binding sites was also studied. The BSA and poziotinib followed a static quenching mechanism as the Stern-Volmer constant showed decrease (7.6 × 104-6.0 × 104) when the temperature increased from 298 K to 310 K. The BSA and poziotinib interaction was spontaneous and enthalpy driven. Involvement of Van der Waals forces and hydrogen bonding in the binding interaction was suggested on the basis of thermodynamic study results. Conformational changes were suggested in the BSA on its interaction with poziotinib based on fluorescence experimental data. The binding constant for BSA-poziotinib showed a maximum decrease in presence of quercetin followed by naringenin, rutin and sinapic acid respectively. Site displacement studies suggested binding of poziotinib site I of BSA.
中文翻译:

泊齐替尼和牛血清白蛋白的结合特性以及槲皮素,芦丁,柚皮苷和芥子酸对其结合相互作用的影响。
血清白蛋白是系统循环中存在的主要转运蛋白,在其他配体存在下,转运配体的能力会受到影响。这种相互作用可以影响某些配体的药效和药代动力学性质。进行了光谱和分子对接研究以了解poziotinib与牛血清白蛋白(BSA)的结合相互作用。此外,还研究了不同类黄酮(槲皮素,芦丁,柚皮素和芥子酸)对从BSA结合位点置换Poziotinib的影响。BSA和poziotinib遵循静态猝灭机制,因为当温度从298 K升高到310 K时,Stern-Volmer常数显示降低(7.6×104-6.0×104)。BSA和poziotinib的相互作用是自发的并且是焓驱动的。根据热力学研究结果,提出范德华力和氢键参与结合相互作用。根据荧光实验数据,BSA与Poziotinib相互作用提示构象变化。BSA-poziotinib的结合常数在槲皮素的存在下显示最大降低,随后分别是柚皮素,芦丁和芥子酸。部位置换研究表明BSA的poziotinib部位I具有约束力。芦丁和芥子酸。部位置换研究表明BSA的poziotinib部位I具有约束力。芦丁和芥子酸。部位置换研究表明BSA的poziotinib部位I具有约束力。
更新日期:2020-04-03
中文翻译:

泊齐替尼和牛血清白蛋白的结合特性以及槲皮素,芦丁,柚皮苷和芥子酸对其结合相互作用的影响。
血清白蛋白是系统循环中存在的主要转运蛋白,在其他配体存在下,转运配体的能力会受到影响。这种相互作用可以影响某些配体的药效和药代动力学性质。进行了光谱和分子对接研究以了解poziotinib与牛血清白蛋白(BSA)的结合相互作用。此外,还研究了不同类黄酮(槲皮素,芦丁,柚皮素和芥子酸)对从BSA结合位点置换Poziotinib的影响。BSA和poziotinib遵循静态猝灭机制,因为当温度从298 K升高到310 K时,Stern-Volmer常数显示降低(7.6×104-6.0×104)。BSA和poziotinib的相互作用是自发的并且是焓驱动的。根据热力学研究结果,提出范德华力和氢键参与结合相互作用。根据荧光实验数据,BSA与Poziotinib相互作用提示构象变化。BSA-poziotinib的结合常数在槲皮素的存在下显示最大降低,随后分别是柚皮素,芦丁和芥子酸。部位置换研究表明BSA的poziotinib部位I具有约束力。芦丁和芥子酸。部位置换研究表明BSA的poziotinib部位I具有约束力。芦丁和芥子酸。部位置换研究表明BSA的poziotinib部位I具有约束力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号