iScience ( IF 4.6 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.isci.2020.101017 Decai Ding 1 , Haiyan Dong 1 , Chuan Wang 1
|
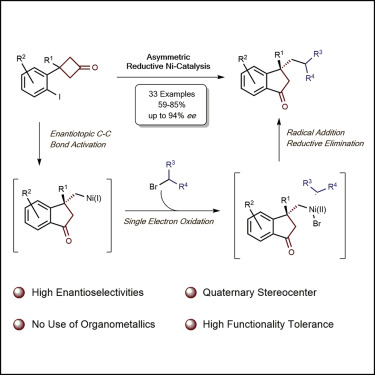
Herein we demonstrate the successful application of reductive strategy in the asymmetric domino ring opening/cross-coupling reaction of prochiral cyclobutanones. Under the catalysis of a chiral nickel complex, various aryl iodide-tethered cyclobutanones were reacted with alkyl bromides as the electrophilic coupling partner, providing a variety of chiral indanones bearing a quaternary stereogenic center in highly enantioselective manner, which can be further converted to diverse benzene-fused cyclic compounds including indane, indene, dihydrocoumarin, and dihydroquinolinone. The preliminary mechanistic investigations support a mechanism involving Ni(I)-mediated enantiotopic C−C σ-bond activation of cyclobutanones as key elementary step in the catalytic cycle.
中文翻译:

通过还原策略镍催化环丁酮的不对称多米诺开环/交叉偶联反应。
在此,我们展示了还原策略在前手性环丁酮的不对称多米诺开环/交叉偶联反应中的成功应用。在手性镍配合物的催化下,各种芳基碘连接的环丁酮与作为亲电偶联伙伴的烷基溴发生反应,以高度对映选择性的方式提供了各种带有季立体中心的手性茚满酮,这些手性茚满酮可以进一步转化为各种苯-稠环化合物,包括茚满、茚、二氢香豆素和二氢喹啉酮。初步的机理研究支持了一种机制,涉及 Ni(I) 介导的环丁酮对映体 C−C σ 键活化,作为催化循环中的关键基本步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号