当前位置:
X-MOL 学术
›
Curr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disruption of EGF Feedback by Intestinal Tumors and Neighboring Cells in Drosophila.
Current Biology ( IF 8.1 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.cub.2020.01.082 Sang Ngo 1 , Jackson Liang 1 , Yu-Han Su 1 , Lucy Erin O'Brien 1
Current Biology ( IF 8.1 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.cub.2020.01.082 Sang Ngo 1 , Jackson Liang 1 , Yu-Han Su 1 , Lucy Erin O'Brien 1
Affiliation
|
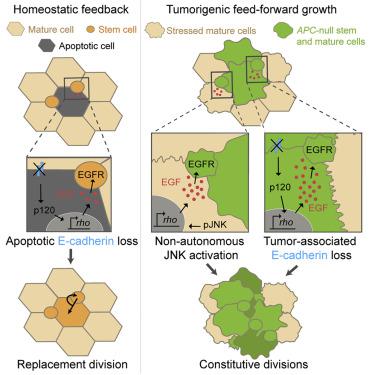
In healthy adult organs, robust feedback mechanisms control cell turnover to enforce homeostatic equilibrium between cell division and death [1, 2]. Nascent tumors must subvert these mechanisms to achieve cancerous overgrowth [3-7]. Elucidating the nature of this subversion can reveal how cancers become established and may suggest strategies to prevent tumor progression. In adult Drosophila intestine, a well-studied model of homeostatic cell turnover, the linchpin of cell equilibrium is feedback control of the epidermal growth factor (EGF) protease Rhomboid (Rho). Expression of Rho in apoptotic cells enables them to secrete EGFs, which stimulate nearby stem cells to undergo replacement divisions [8]. As in mammals, loss of adenomatous polyposis coli (APC) causes Drosophila intestinal stem cells to form adenomas [9]. Here, we demonstrate that Drosophila APC-/- tumors trigger widespread Rho expression in non-apoptotic cells, resulting in chronic EGF signaling. Initially, nascent APC-/- tumors induce rho in neighboring wild-type cells via acute, non-autonomous activation of Jun N-terminal kinase (JNK). During later growth and multilayering, APC-/- tumors induce rho in tumor cells by autonomous downregulation of E-cadherin (E-cad) and consequent activity of p120-catenin. This sequential dysregulation of tumor non-autonomous and -autonomous EGF signaling converts tissue-level feedback into feed-forward activation that drives cancerous overgrowth. Because Rho, EGF receptor (EGFR), and E-cad are associated with colorectal cancer in humans [10-17], our findings may shed light on how human colorectal tumors progress.
中文翻译:

果蝇肠道肿瘤和邻近细胞对 EGF 反馈的破坏。
在健康的成人器官中,强大的反馈机制控制细胞更新,以强制细胞分裂和死亡之间的稳态平衡 [1, 2]。新生肿瘤必须颠覆这些机制才能实现癌性过度生长[3-7]。阐明这种颠覆的本质可以揭示癌症是如何形成的,并可能提出预防肿瘤进展的策略。在成年果蝇肠道中,这是一种经过充分研究的稳态细胞更新模型,细胞平衡的关键是表皮生长因子 (EGF) 蛋白酶菱形 (Rho) 的反馈控制。 Rho 在凋亡细胞中的表达使它们能够分泌 EGF,从而刺激附近的干细胞进行更替分裂 [8]。与哺乳动物一样,腺瘤性结肠息肉病 (APC) 的丧失会导致果蝇肠道干细胞形成腺瘤 [9]。在这里,我们证明果蝇 APC-/- 肿瘤触发非凋亡细胞中广泛的 Rho 表达,从而导致慢性 EGF 信号传导。最初,新生的 APC-/- 肿瘤通过 Jun N 末端激酶 (JNK) 的急性、非自主激活在邻近的野生型细胞中诱导 rho。在随后的生长和多层化过程中,APC-/- 肿瘤通过 E-钙粘蛋白 (E-cad) 的自主下调以及随后的 p120-连环蛋白活性诱导肿瘤细胞中的 rho。肿瘤非自主和自主 EGF 信号传导的连续失调将组织水平反馈转化为前馈激活,从而驱动癌性过度生长。由于 Rho、EGF 受体 (EGFR) 和 E-cad 与人类结直肠癌相关 [10-17],因此我们的研究结果可能有助于了解人类结直肠肿瘤的进展。
更新日期:2020-04-20
中文翻译:

果蝇肠道肿瘤和邻近细胞对 EGF 反馈的破坏。
在健康的成人器官中,强大的反馈机制控制细胞更新,以强制细胞分裂和死亡之间的稳态平衡 [1, 2]。新生肿瘤必须颠覆这些机制才能实现癌性过度生长[3-7]。阐明这种颠覆的本质可以揭示癌症是如何形成的,并可能提出预防肿瘤进展的策略。在成年果蝇肠道中,这是一种经过充分研究的稳态细胞更新模型,细胞平衡的关键是表皮生长因子 (EGF) 蛋白酶菱形 (Rho) 的反馈控制。 Rho 在凋亡细胞中的表达使它们能够分泌 EGF,从而刺激附近的干细胞进行更替分裂 [8]。与哺乳动物一样,腺瘤性结肠息肉病 (APC) 的丧失会导致果蝇肠道干细胞形成腺瘤 [9]。在这里,我们证明果蝇 APC-/- 肿瘤触发非凋亡细胞中广泛的 Rho 表达,从而导致慢性 EGF 信号传导。最初,新生的 APC-/- 肿瘤通过 Jun N 末端激酶 (JNK) 的急性、非自主激活在邻近的野生型细胞中诱导 rho。在随后的生长和多层化过程中,APC-/- 肿瘤通过 E-钙粘蛋白 (E-cad) 的自主下调以及随后的 p120-连环蛋白活性诱导肿瘤细胞中的 rho。肿瘤非自主和自主 EGF 信号传导的连续失调将组织水平反馈转化为前馈激活,从而驱动癌性过度生长。由于 Rho、EGF 受体 (EGFR) 和 E-cad 与人类结直肠癌相关 [10-17],因此我们的研究结果可能有助于了解人类结直肠肿瘤的进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号