Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.chembiol.2020.03.003 Chung Sub Kim 1 , Jhe-Hao Li 1 , Brenden Barco 2 , Hyun Bong Park 1 , Alexandra Gatsios 1 , Ashiti Damania 2 , Rurun Wang 3 , Thomas P Wyche 3 , Grazia Piizzi 3 , Nicole K Clay 2 , Jason M Crawford 4
|
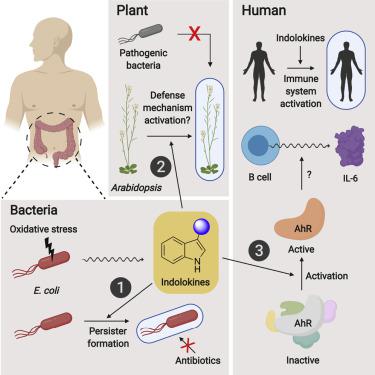
Escherichia coli broadly colonize the intestinal tract of humans and produce a variety of small molecule signals. However, many of these small molecules remain unknown. Here, we describe a family of widely distributed bacterial metabolites termed the “indolokines.” In E. coli, the indolokines are upregulated in response to a redox stressor via aspC and tyrB transaminases. Although indolokine 1 represents a previously unreported metabolite, four of the indolokines (2–5) were previously shown to be derived from indole-3-carbonyl nitrile (ICN) in the plant pathogen defense response. We show that the indolokines are produced in a convergent evolutionary manner relative to plants, enhance E. coli persister cell formation, outperform ICN protection in an Arabidopsis thaliana-Pseudomonas syringae infection model, trigger a hallmark plant innate immune response, and activate distinct immunological responses in primary human tissues. Our molecular studies link a family of cellular stress-induced metabolites to defensive responses across bacteria, plants, and humans.
中文翻译:

细胞应激上调大肠杆菌中的吲哚信号代谢物。
大肠杆菌广泛定植于人类肠道并产生多种小分子信号。然而,许多这些小分子仍然未知。在这里,我们描述了一个广泛分布的细菌代谢物家族,称为“吲哚活素”。在大肠杆菌中,吲哚因子通过aspC和tyrB转氨酶响应氧化还原应激源而上调。尽管吲哚活素1是一种先前未报道的代谢物,但先前已证明其中四种吲哚活素 ( 2-5 ) 源自植物病原体防御反应中的吲哚-3-羰基腈 (ICN)。我们表明,吲哚活素以相对于植物的趋同进化方式产生,增强了大肠杆菌持久细胞的形成,在拟南芥-丁香假单胞菌感染模型中优于ICN保护,触发标志性植物先天免疫反应,并激活不同的免疫反应在人体初级组织中。我们的分子研究将一系列细胞应激诱导的代谢物与细菌、植物和人类的防御反应联系起来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号