Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.elecom.2020.106720 Rene Böttcher , Adriana Ispas , Andreas Bund
|
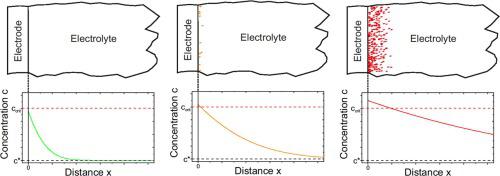
The anodic dissolution of aluminum in Lewis acidic ionic liquids consisting of AlCl3 and 1-ethyl-3-methylimidazolium chloride was studied using linear sweep and cyclic voltammetry, an electrochemical quartz crystal microbalance (EQCM) and chronopotentiometry at ambient temperature. Anodic passivation of the working electrode was observed in a 2:1 electrolyte while no passivation was found in a 1.5:1 electrolyte. Chronopotentiometry proves the passivation to be caused by local solidification of the electrolyte due to an increase in the aluminum concentration near the anode. EQCM data support these results.
中文翻译:

[EMIm] Cl基离子液体中铝的阳极溶解和阳极钝化
使用线性扫描和循环伏安法,电化学石英晶体微量天平(EQCM)和计时电位法在环境温度下研究了铝在由AlCl 3和1-乙基-3-甲基咪唑鎓氯化物组成的Lewis酸性离子液体中的阳极溶解。在2:1电解液中观察到工作电极的阳极钝化,而在1.5:1电解液中未发现钝化。计时电位法证明钝化是由于阳极附近铝浓度增加而引起的电解质的局部固化引起的。EQCM数据支持这些结果。









































 京公网安备 11010802027423号
京公网安备 11010802027423号