当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
hERG toxicity assessment: Useful guidelines for drug design.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.ejmech.2020.112290 Amanda Garrido 1 , Alban Lepailleur 1 , Serge M Mignani 2 , Patrick Dallemagne 1 , Christophe Rochais 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.ejmech.2020.112290 Amanda Garrido 1 , Alban Lepailleur 1 , Serge M Mignani 2 , Patrick Dallemagne 1 , Christophe Rochais 1
Affiliation
|
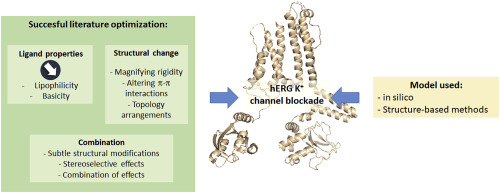
All along the drug development process, one of the most frequent adverse side effects, leading to the failure of drugs, is the cardiac arrhythmias. Such failure is mostly related to the capacity of the drug to inhibit the human ether-à-go-go-related gene (hERG) cardiac potassium channel. The early identification of hERG inhibition properties of biological active compounds has focused most of attention over the years. In order to prevent the cardiac side effects, a great number of in silico, in vitro and in vivo assays have been performed. The main goal of these studies is to understand the reasons of these effects, and then to give information or instructions to scientists involved in drug development to avoid the cardiac side effects. To evaluate anticipated cardiovascular effects, early evaluation of hERG toxicity has been strongly recommended for instance by the regulatory agencies such as U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA). Thus, following an initial screening of a collection of compounds to find hits, a great number of pharmacomodulation studies on the novel identified chemical series need to be performed including activity evaluation towards hERG. We provide in this concise review clear guidelines, based on described examples, illustrating successful optimization process to avoid hERG interactions as cases studies and to spur scientists to develop safe drugs.
中文翻译:

hERG毒性评估:药物设计的有用指南。
在整个药物开发过程中,导致心律失常的最常见不良副作用之一是导致药物衰竭。这种失败主要与药物抑制人类以太相关基因(hERG)心脏钾通道的能力有关。多年来,对生物活性化合物的hERG抑制特性的早期鉴定已引起了大多数关注。为了防止心脏副作用,已经进行了大量计算机,体外和体内测定。这些研究的主要目的是了解这些作用的原因,然后向参与药物开发的科学家提供信息或指导,以避免心脏副作用。为了评估预期的心血管作用,例如,美国食品药品监督管理局(FDA)和欧洲药品管理局(EMA)等监管机构强烈建议对hERG毒性进行早期评估。因此,在初步筛选出一系列化合物以寻找结果之后,需要对新鉴定的化学系列进行大量的药物调节研究,包括对hERG的活性评估。我们在简明扼要的审查中提供了基于所描述示例的清晰指南,阐明了成功的优化过程,以避免案例研究中的hERG相互作用,并鼓励科学家开发安全的药物。需要对新鉴定出的化学系列进行大量药物调节研究,包括对hERG的活性评估。我们在简明扼要的审查中提供了基于所描述示例的清晰指南,阐明了成功的优化过程,以避免案例研究中的hERG相互作用,并鼓励科学家开发安全的药物。需要对新鉴定出的化学系列进行大量药物调节研究,包括对hERG的活性评估。我们在简明扼要的审查中提供了基于所描述示例的清晰指南,阐明了成功的优化过程,以避免案例研究中的hERG相互作用,并鼓励科学家开发安全的药物。
更新日期:2020-04-03
中文翻译:

hERG毒性评估:药物设计的有用指南。
在整个药物开发过程中,导致心律失常的最常见不良副作用之一是导致药物衰竭。这种失败主要与药物抑制人类以太相关基因(hERG)心脏钾通道的能力有关。多年来,对生物活性化合物的hERG抑制特性的早期鉴定已引起了大多数关注。为了防止心脏副作用,已经进行了大量计算机,体外和体内测定。这些研究的主要目的是了解这些作用的原因,然后向参与药物开发的科学家提供信息或指导,以避免心脏副作用。为了评估预期的心血管作用,例如,美国食品药品监督管理局(FDA)和欧洲药品管理局(EMA)等监管机构强烈建议对hERG毒性进行早期评估。因此,在初步筛选出一系列化合物以寻找结果之后,需要对新鉴定的化学系列进行大量的药物调节研究,包括对hERG的活性评估。我们在简明扼要的审查中提供了基于所描述示例的清晰指南,阐明了成功的优化过程,以避免案例研究中的hERG相互作用,并鼓励科学家开发安全的药物。需要对新鉴定出的化学系列进行大量药物调节研究,包括对hERG的活性评估。我们在简明扼要的审查中提供了基于所描述示例的清晰指南,阐明了成功的优化过程,以避免案例研究中的hERG相互作用,并鼓励科学家开发安全的药物。需要对新鉴定出的化学系列进行大量药物调节研究,包括对hERG的活性评估。我们在简明扼要的审查中提供了基于所描述示例的清晰指南,阐明了成功的优化过程,以避免案例研究中的hERG相互作用,并鼓励科学家开发安全的药物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号