当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
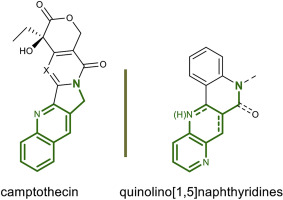
Synthesis of novel hybrid quinolino[4,3-b][1,5]naphthyridines and quinolino[4,3-b][1,5]naphthyridin-6(5H)-one derivatives and biological evaluation as topoisomerase I inhibitors and antiproliferatives.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.ejmech.2020.112292 Endika Martín-Encinas 1 , Asier Selas 2 , Cinzia Tesauro 3 , Gloria Rubiales 1 , Birgitta R Knudsen 3 , Francisco Palacios 1 , Concepción Alonso 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.ejmech.2020.112292 Endika Martín-Encinas 1 , Asier Selas 2 , Cinzia Tesauro 3 , Gloria Rubiales 1 , Birgitta R Knudsen 3 , Francisco Palacios 1 , Concepción Alonso 1
Affiliation
|
The topoisomerase I enzymatic inhibition of hybrid quinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridines and quinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridin-6(5H)-ones was investigated. First, the synthesis of these fused compounds was performed by intramolecular [4 + 2]-cycloaddition reaction of functionalized aldimines obtained by the condensation of 3-aminopyridine and unsaturated aldehydes affording corresponding hybrid 5-tosylhexahydroquinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridine and tetrahydroquinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridin-6(5H)-one compounds with good to high general yields. Subsequent dehydrogenation led to the corresponding more unsaturated dihydro (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridine and (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridin-6(5H)-one derivatives in quantitative yields. The new polycyclic products show excellent-good activity as topoisomerase I (TopI) inhibitors that lead to TopI induced nicking of plasmids. This is consistent with the compounds acting as TopI poisons resulting in the accumulation of trapped cleavage complexes in the DNA. The cytotoxic effect on cell lines A549, SKOV3 and on non-cancerous MRC5 was also screened. Tetrahydroquinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridin-6(5H)-one 9 resulted the most cytotoxic compound with IC50 values of 3.25 ± 0.91 μM and 2.08 ± 1.89 μM against the A549 cell line and the SKOV3 cell line, respectively. Also, hexahydroquinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridine 8a and dihydroquinolino [4,3-b] (Siegel et al., 2013; Antony et al., 2003) [1,5]naphthyridine 10a showed good cytotoxicity against these cell lines. None of the compounds presented cytotoxic effects against non-malignant pulmonary fibroblasts (MRC-5).
中文翻译:

新型杂喹啉[4,3-b] [1,5]萘啶和喹啉[4,3-b] [1,5]萘啶-6(5H)-one衍生物的合成及作为拓扑异构酶I抑制剂和抗增殖剂的生物学评价。
拓扑异构酶I对杂合喹啉基[4,3-b]的酶促抑制作用(Siegel等,2013; Antony等人,2003)[1,5]萘啶和喹啉基[4,3-b](Siegel等。 ,2013; Antony等,2003)研究了[1,5]萘啶-6(5H)-一。首先,通过3-氨基吡啶和不饱和醛的缩合反应得到的官能化醛亚胺的分子内[4 + 2]-环加成反应,得到相应的杂合的5-甲苯基六氢喹啉[4,3-b](Siegel等,等人,2013;安东尼等人,2003)[1,5]萘啶和四氢喹啉基[4,3-b](Siegel等人,2013;安东尼等人,2003)[1,5]萘啶-6 (5H)-一化合物,具有良好至高的一般收率。随后的脱氢反应导致相应的不饱和二氢含量更高(Siegel等,2013; Antony等,2003)[1,5]萘啶和(Siegel等人,2013; Antony等人,2003)[1,5]萘啶-6(5H)-一衍生物的定量收率。这种新的多环产物作为拓扑异构酶I(TopI)抑制剂具有极好的优良活性,可导致TopI诱导质粒产生切口。这与充当TopI毒物的化合物相一致,导致捕获的切割复合物在DNA中积累。还筛选了对细胞系A549,SKOV3和对非癌性MRC5的细胞毒性作用。四氢喹啉[4,3-b](Siegel等,2013; Antony等,2003)[1,5]萘啶-6(5H)-1产生的细胞毒性最大,IC50值为3.25±0.91μM对A549细胞系和SKOV3细胞系分别为2.08±1.89μM。另外,六氢喹啉[4,3-b](Siegel等,2013; Antony等,2003)[1,5]萘啶8a和二氢喹啉[4,3-b](Siegel等,2013; Antony等,2003)[1,5]萘啶10a对这些细胞系表现出良好的细胞毒性。没有一种化合物对非恶性肺成纤维细胞(MRC-5)表现出细胞毒性作用。
更新日期:2020-04-03
中文翻译:

新型杂喹啉[4,3-b] [1,5]萘啶和喹啉[4,3-b] [1,5]萘啶-6(5H)-one衍生物的合成及作为拓扑异构酶I抑制剂和抗增殖剂的生物学评价。
拓扑异构酶I对杂合喹啉基[4,3-b]的酶促抑制作用(Siegel等,2013; Antony等人,2003)[1,5]萘啶和喹啉基[4,3-b](Siegel等。 ,2013; Antony等,2003)研究了[1,5]萘啶-6(5H)-一。首先,通过3-氨基吡啶和不饱和醛的缩合反应得到的官能化醛亚胺的分子内[4 + 2]-环加成反应,得到相应的杂合的5-甲苯基六氢喹啉[4,3-b](Siegel等,等人,2013;安东尼等人,2003)[1,5]萘啶和四氢喹啉基[4,3-b](Siegel等人,2013;安东尼等人,2003)[1,5]萘啶-6 (5H)-一化合物,具有良好至高的一般收率。随后的脱氢反应导致相应的不饱和二氢含量更高(Siegel等,2013; Antony等,2003)[1,5]萘啶和(Siegel等人,2013; Antony等人,2003)[1,5]萘啶-6(5H)-一衍生物的定量收率。这种新的多环产物作为拓扑异构酶I(TopI)抑制剂具有极好的优良活性,可导致TopI诱导质粒产生切口。这与充当TopI毒物的化合物相一致,导致捕获的切割复合物在DNA中积累。还筛选了对细胞系A549,SKOV3和对非癌性MRC5的细胞毒性作用。四氢喹啉[4,3-b](Siegel等,2013; Antony等,2003)[1,5]萘啶-6(5H)-1产生的细胞毒性最大,IC50值为3.25±0.91μM对A549细胞系和SKOV3细胞系分别为2.08±1.89μM。另外,六氢喹啉[4,3-b](Siegel等,2013; Antony等,2003)[1,5]萘啶8a和二氢喹啉[4,3-b](Siegel等,2013; Antony等,2003)[1,5]萘啶10a对这些细胞系表现出良好的细胞毒性。没有一种化合物对非恶性肺成纤维细胞(MRC-5)表现出细胞毒性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号