Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.tetlet.2020.151912 Xi Chen , Muhua Wang , Xinying Zhang , Xuesen Fan
|
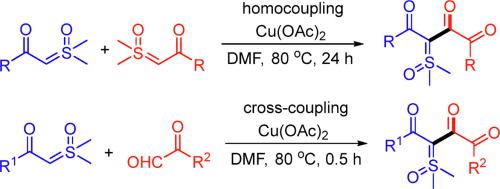
A novel synthesis of functionalized 1,2,4-triones via an oxidative homo-coupling of β-keto sulfoxonium ylides is presented. Preliminary mechanistic study reveals that the formation of the 1,2,4-trione product involves the in situ generation of an α-keto aldehyde intermediate via Cu(II)-promoted oxidative decomposition of β-keto sulfoxonium ylide followed by its coupling with the second molecule of ylide. Based on this intrinsic mechanism, a formal cross-coupling reaction was designed and successfully realized, from which more diversely functionalized 1,2,4-triones were synthesized with high efficiency. Compared with literature methods, notable features of this novel synthetic protocol include easily accessible and safe substrates, excellent functional group tolerance, convenient procedure, mild reaction conditions, and ready scalability.
中文翻译:

通过β-酮基亚砜基磺酸盐的均相和交叉偶联反应新颖合成功能多样的1,2,4-三酮
提出了一种新的功能化的1,2,4-三酮经β-酮基亚砜基鎓盐的氧化均偶联合成。初步的机理研究表明,1,2,4-三酮产物的形成涉及通过Cu(II)促进β-酮基亚砜sulf盐的氧化分解,然后将其与环戊二烯偶联,原位生成α-酮醛中间体。第二个叶立德分子。基于这种内在机理,设计并成功实现了正式的交叉偶联反应,由此可以高效地合成更多官能化的1,2,4-三酮。与文献方法相比,这种新颖的合成方案的显着特征包括易于获取和安全的底物,出色的官能团耐受性,简便的操作程序,温和的反应条件以及易于扩展的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号