当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling the Sterol-Binding Domain of Aster-A Provides Insight into Its Multiligand Specificity.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jcim.0c00086 Laust Moesgaard 1 , Peter Reinholdt 1 , Daniel Wüstner 2 , Jacob Kongsted 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jcim.0c00086 Laust Moesgaard 1 , Peter Reinholdt 1 , Daniel Wüstner 2 , Jacob Kongsted 1
Affiliation
|
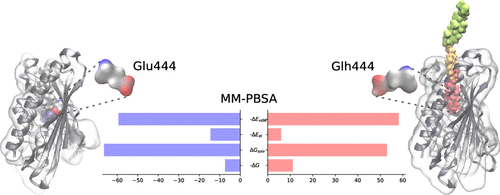
Intracellular transport of cholesterol and related sterols relies to a large degree on nonvesicular mechanisms, which are only partly understood at the molecular level. Aster proteins belonging to the Lam family of sterol transfer proteins have recently been identified as important catalysts of nonvesicular sterol exchange between the plasma membrane (PM) and endoplasmic reticulum (ER). Here, we used a range of computational tools to study the molecular mechanisms underlying sterol binding as well as multisterol ligand specificity of Aster-A. Our study focused primarily on gaining atomistic insight into the bound ligand-protein complex and was, on this basis, performed in the absence of any membrane. Molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) calculations provide a rationale for the experimentally found ranking of binding affinities of various sterols to Aster-A. In particular, the polarity of the sterols and the length of their alkyl chain could be identified as being critical determinants of ligand affinity. A Gibbs free energy decomposition identified a charged residue, Glu444, at the base of the binding pose as an important control point for sterol binding. Removing its net charge via protonation was found to cause significant changes to the environment surrounding this residue. In addition, the protonation of Glu444 was found to be paralleled by a large redistribution of molecular flexibility in the Aster domain. This finding was supplemented by multiple branched adaptive steered molecular dynamics (MB-ASMD) simulations by which we defined a possible molecular path for sterol release and demonstrated the importance of Glu444 in this process.
中文翻译:

对Aster-A的甾醇结合域进行建模可深入了解其多配体特异性。
胆固醇和相关固醇的细胞内运输在很大程度上取决于非囊泡机制,这在分子水平上仅是部分理解。最近,已确认属于Lam固醇转移蛋白家族的Aster蛋白是质膜(PM)与内质网(ER)之间非囊状固醇交换的重要催化剂。在这里,我们使用了多种计算工具来研究固醇结合以及Aster-A的多固醇配体特异性的分子机制。我们的研究主要集中在对结合的配体-蛋白质复合物的原子分析上,并在没有任何膜的情况下进行。分子力学泊松玻耳兹曼表面积(MM-PBSA)计算为各种固醇与Aster-A的结合亲和力的实验发现排名提供了理论依据。特别地,固醇的极性及其烷基链的长度可以被认为是配体亲和力的关键决定因素。吉布斯自由能分解法确定了在结合位点的基础上,带电残基Glu444是固醇结合的重要控制点。发现通过质子化去除其净电荷会导致该残留物周围的环境发生重大变化。另外,发现Glu444的质子化与Aster结构域中分子柔性的大的重新分布平行。
更新日期:2020-04-01
中文翻译:

对Aster-A的甾醇结合域进行建模可深入了解其多配体特异性。
胆固醇和相关固醇的细胞内运输在很大程度上取决于非囊泡机制,这在分子水平上仅是部分理解。最近,已确认属于Lam固醇转移蛋白家族的Aster蛋白是质膜(PM)与内质网(ER)之间非囊状固醇交换的重要催化剂。在这里,我们使用了多种计算工具来研究固醇结合以及Aster-A的多固醇配体特异性的分子机制。我们的研究主要集中在对结合的配体-蛋白质复合物的原子分析上,并在没有任何膜的情况下进行。分子力学泊松玻耳兹曼表面积(MM-PBSA)计算为各种固醇与Aster-A的结合亲和力的实验发现排名提供了理论依据。特别地,固醇的极性及其烷基链的长度可以被认为是配体亲和力的关键决定因素。吉布斯自由能分解法确定了在结合位点的基础上,带电残基Glu444是固醇结合的重要控制点。发现通过质子化去除其净电荷会导致该残留物周围的环境发生重大变化。另外,发现Glu444的质子化与Aster结构域中分子柔性的大的重新分布平行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号