当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of Cu(II) and Mixed-Valence Cu(I)Cu(II) Clusters Supported by Pyridylamide Ligands.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.inorgchem.0c00008 Joseph D Schneider 1 , Brett A Smith 2 , Grant A Williams 1 , Douglas R Powell 3 , Felio Perez 4 , Gerard T Rowe 2 , Lei Yang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.inorgchem.0c00008 Joseph D Schneider 1 , Brett A Smith 2 , Grant A Williams 1 , Douglas R Powell 3 , Felio Perez 4 , Gerard T Rowe 2 , Lei Yang 1
Affiliation
|
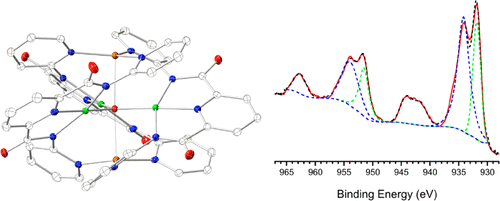
A group of copper complexes supported by polydentate pyridylamide ligands H2bpda and H2ppda were synthesized and characterized. The two Cu(II) dimers [CuII2(Hbpda)2(ClO4)2] (1) and [CuII2(ppda)2(DMF)2] (2) were constructed by using neutral ligands to react with Cu(II) salts. Although the dimers showed similar structural features, the second-sphere interactions affect the structures differently. With the application of Et3N, the tetranuclear cluster (HNEt3)[CuII4(bpda)2(μ3-OH)2(ClO4)(DMF)3](ClO4)2 (3) and hexanuclear cluster (HNEt3)2[CuII6(ppda)6(H2O)2(CH3OH)2](ClO4)2 (4) were prepared under similar reaction conditions. The symmetrical and unsymmetrical arrangement of the ligand donors in ligands H2bpda and H2ppda led to the dramatic conformation difference of the two Cu(II) complexes. As part of our effort to explore mixed-valence copper chemistry, the triple-decker pentanuclear cluster [CuII3CuI2(bpda)3(μ3-O)] (5) was prepared. XPS examination demonstrated the localized mixed-valence properties of complex 5. Magnetic studies of the clusters with EPR evidence showed either weak ferromagnetic or antiferromagnetic interactions among copper centers. Due to the trigonal-planar conformation of the trinuclear Cu(II) motif with the μ3-O center, complex 5 exhibits geometric spin frustration and engages in antisymmetric exchange interactions. DFT calculations were also performed to better interpret spectroscopic evidence and understand the electronic structures, especially the mixed-valence nature of complex 5.
中文翻译:

吡啶酰胺配体支撑的Cu(II)和混合价Cu(I)Cu(II)团簇的合成与表征。
合成并表征了由多齿吡啶酰胺配体H2bpda和H2ppda支撑的一组铜络合物。通过使用中性配体与Cu(II)盐反应构建了两个Cu(II)二聚体[CuII2(Hbpda)2(ClO4)2](1)和[CuII2(ppda)2(DMF)2](2)。 。尽管二聚体显示出相似的结构特征,但第二球相互作用对结构的影响不同。随着Et3N的应用,四核簇(HNEt3)[CuII4(bpda)2(μ3-OH)2(ClO4)(DMF)3](ClO4)2(3)和六核簇(HNEt3)2 [CuII6(ppda)在相似的反应条件下制备)6(H 2 O)2(CH 3 OH)2](ClO 4)2(4)。配体H2bpda和H2ppda中配体供体的对称和不对称排列导致两种Cu(II)配合物的构象差异显着。为了探索混合价铜化学,制备了三层五核簇[CuII3CuI2(bpda)3(μ3-O)](5)。XPS检查证明了配合物5的局部混合价性质。具有EPR证据的簇的磁研究表明,铜中心之间的铁磁相互作用弱或反铁磁相互作用。由于三核Cu(II)基序具有μ3-O中心的三角平面构型,复合物5表现出几何自旋受阻,并参与反对称交换相互作用。还进行了DFT计算,以更好地解释光谱证据并了解电子结构,尤其是配合物5的混合价性质。具有EPR证据的簇的磁研究表明,铜中心之间的铁磁相互作用弱或反铁磁相互作用。由于三核Cu(II)基序具有μ3-O中心的三角平面构型,复合物5表现出几何自旋受阻,并参与反对称交换相互作用。还进行了DFT计算,以更好地解释光谱证据并了解电子结构,尤其是配合物5的混合价性质。具有EPR证据的簇的磁研究表明,铜中心之间的铁磁相互作用弱或反铁磁相互作用。由于三核Cu(II)基序具有μ3-O中心的三角平面构型,复合物5表现出几何自旋受阻,并参与反对称交换相互作用。还进行了DFT计算,以更好地解释光谱证据并了解电子结构,尤其是配合物5的混合价性质。
更新日期:2020-04-24
中文翻译:

吡啶酰胺配体支撑的Cu(II)和混合价Cu(I)Cu(II)团簇的合成与表征。
合成并表征了由多齿吡啶酰胺配体H2bpda和H2ppda支撑的一组铜络合物。通过使用中性配体与Cu(II)盐反应构建了两个Cu(II)二聚体[CuII2(Hbpda)2(ClO4)2](1)和[CuII2(ppda)2(DMF)2](2)。 。尽管二聚体显示出相似的结构特征,但第二球相互作用对结构的影响不同。随着Et3N的应用,四核簇(HNEt3)[CuII4(bpda)2(μ3-OH)2(ClO4)(DMF)3](ClO4)2(3)和六核簇(HNEt3)2 [CuII6(ppda)在相似的反应条件下制备)6(H 2 O)2(CH 3 OH)2](ClO 4)2(4)。配体H2bpda和H2ppda中配体供体的对称和不对称排列导致两种Cu(II)配合物的构象差异显着。为了探索混合价铜化学,制备了三层五核簇[CuII3CuI2(bpda)3(μ3-O)](5)。XPS检查证明了配合物5的局部混合价性质。具有EPR证据的簇的磁研究表明,铜中心之间的铁磁相互作用弱或反铁磁相互作用。由于三核Cu(II)基序具有μ3-O中心的三角平面构型,复合物5表现出几何自旋受阻,并参与反对称交换相互作用。还进行了DFT计算,以更好地解释光谱证据并了解电子结构,尤其是配合物5的混合价性质。具有EPR证据的簇的磁研究表明,铜中心之间的铁磁相互作用弱或反铁磁相互作用。由于三核Cu(II)基序具有μ3-O中心的三角平面构型,复合物5表现出几何自旋受阻,并参与反对称交换相互作用。还进行了DFT计算,以更好地解释光谱证据并了解电子结构,尤其是配合物5的混合价性质。具有EPR证据的簇的磁研究表明,铜中心之间的铁磁相互作用弱或反铁磁相互作用。由于三核Cu(II)基序具有μ3-O中心的三角平面构型,复合物5表现出几何自旋受阻,并参与反对称交换相互作用。还进行了DFT计算,以更好地解释光谱证据并了解电子结构,尤其是配合物5的混合价性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号