当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.accounts.0c00032 Justin Diccianni 1 , Qiao Lin 1 , Tianning Diao 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.accounts.0c00032 Justin Diccianni 1 , Qiao Lin 1 , Tianning Diao 1
Affiliation
|
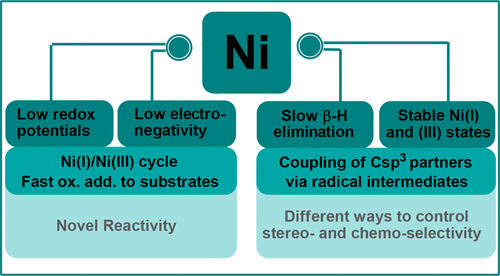
ConspectusNickel complexes exhibit distinct properties from other group 10 metals, including a small nuclear radius, high paring energy, low electronegativity, and low redox potentials. These properties enable Ni catalysts to accommodate and stabilize paramagnetic intermediates, access radical pathways, and undergo slow β-H elimination. Our research program investigates how each of these fundamental attributes impact the catalytic properties of Ni, in particular in the context of alkene functionalization.Alkenes are versatile functional groups, but stereoselective carbofunctionalization reactions of alkenes have been underdeveloped. This challenge may derive from the difficulty of controlling selectivity via traditional two-electron migratory insertion pathways. Ni catalysts could lead to different stereodetermining steps via radical mechanisms, allowing access to molecular scaffolds that are otherwise difficult to prepare. For example, an asymmetric alkene diarylation reaction developed by our group relies upon the radical properties of Ni(III) intermediates to control the enantioselectivity and give access to a library of chiral α,α,β-triarylethane molecules with biological activity.Mechanistic studies on a two-component reductive 1,2-difunctionalization reaction have shed light on the origin of the cross-electrophile selectivity, as C sp2 and C sp3 electrophiles are independently activated at Ni(I) via two-electron and radical pathways, respectively. Catalyst reduction has been identified to be the turnover-limiting step in this system. A closer investigation of the radical formation step using a (Xantphos)Ni(I)Ar model complex reveals that Ni(I) initiates radical formation via a concerted halogen-abstraction pathway.The low redox potentials of Ni have allowed us to develop a reductive, trans-selective diene cyclization, wherein a classic two-electron mechanism operates on a Ni(I)/Ni(III) platform, accounting for the chemo- and stereoselectivity. This reaction has found applications in the efficient synthesis of pharmaceutically relevant molecules, such as 3,4-dimethylgababutin.The tendency of Ni to undergo one-electron redox processes prompted us to explore dinuclear Ni-mediated bond formations. These studies provide insight into Ni-Ni bonding and how two metal centers react cooperatively to promote C-C, C-X, and N-N bond forming reductive elimination.Finally, isolation of β-agostic Ni and Pd complexes has allowed for X-ray and neutron diffraction characterization of these highly reactive molecules. The bonding parameters serve as unambiguous evidence for β-agostic interactions and help rationalize the slower β-H elimination at Ni relative to Pd. Overall, our research has elucidated the fundamental properties of Ni complexes in several contexts. Greater mechanistic understanding facilitates catalyst design and helps rationalize the reactivity and selectivity in Ni-catalyzed alkene functionalization reactions.
中文翻译:

镍催化的偶联反应机理及其在烯烃官能化中的应用。
结论镍配合物表现出与其他第10组金属不同的特性,包括较小的核半径,较高的削皮能,较低的电负性和较低的氧化还原电势。这些特性使Ni催化剂能够适应和稳定顺磁性中间体,进入自由基通道并缓慢地进行β-H消除。我们的研究计划研究了这些基本属性如何影响Ni的催化性能,特别是在烯烃官能化的情况下。烯烃是多官能团,但烯烃的立体选择性碳官能化反应尚不完善。这一挑战可能源于通过传统的两电子迁移插入途径控制选择性的困难。Ni催化剂可能通过自由基机理导致不同的立体确定步骤,允许接触否则难以制备的分子支架。例如,我们小组开发的不对称烯烃二芳基化反应依赖于Ni(III)中间体的自由基性质来控制对映选择性并提供具有生物活性的手性α,α,β-三芳基乙烷分子库。两组分的还原性1,2-双官能化反应为交叉亲电子试剂的选择性提供了线索,因为C sp2和C sp3亲电子试剂分别通过二电子和自由基途径在Ni(I)上独立活化。在该系统中,催化剂还原已被确定为限制周转的步骤。使用(Xantphos)Ni(I)Ar模型配合物仔细研究自由基形成步骤发现,Ni(I)通过一致的卤素吸收途径引发自由基形成.Ni的低氧化还原电势使我们能够开发还原性,反式选择性二烯环化反应,其中经典的两电子机制在Ni(I)/ Ni(III)平台上起作用,这说明了化学和立体选择性。该反应已发现在药物相关分子(例如3,4-二甲基加巴布丁)的有效合成中的应用。Ni经历单电子氧化还原过程的趋势促使我们探索双核Ni介导的键形成。这些研究提供了关于Ni-Ni键以及两个金属中心如何协同反应以促进CC,CX和NN键形成还原消除的见解。分离β-声镍和钯配合物可以对这些高反应性分子进行X射线和中子衍射表征。键合参数是β-声波相互作用的明确证据,有助于合理地消除相对于Pd的Ni处较慢的β-H消除。总的来说,我们的研究阐明了在多种情况下镍络合物的基本性质。更好的机械理解有助于催化剂设计,并有助于合理化Ni催化的烯烃官能化反应中的反应性和选择性。我们的研究阐明了在多种情况下镍络合物的基本性质。更好的机械理解有助于催化剂设计,并有助于合理化Ni催化的烯烃官能化反应的反应性和选择性。我们的研究阐明了在多种情况下镍络合物的基本性质。更好的机械理解有助于催化剂设计,并有助于合理化Ni催化的烯烃官能化反应中的反应性和选择性。
更新日期:2020-04-23
中文翻译:

镍催化的偶联反应机理及其在烯烃官能化中的应用。
结论镍配合物表现出与其他第10组金属不同的特性,包括较小的核半径,较高的削皮能,较低的电负性和较低的氧化还原电势。这些特性使Ni催化剂能够适应和稳定顺磁性中间体,进入自由基通道并缓慢地进行β-H消除。我们的研究计划研究了这些基本属性如何影响Ni的催化性能,特别是在烯烃官能化的情况下。烯烃是多官能团,但烯烃的立体选择性碳官能化反应尚不完善。这一挑战可能源于通过传统的两电子迁移插入途径控制选择性的困难。Ni催化剂可能通过自由基机理导致不同的立体确定步骤,允许接触否则难以制备的分子支架。例如,我们小组开发的不对称烯烃二芳基化反应依赖于Ni(III)中间体的自由基性质来控制对映选择性并提供具有生物活性的手性α,α,β-三芳基乙烷分子库。两组分的还原性1,2-双官能化反应为交叉亲电子试剂的选择性提供了线索,因为C sp2和C sp3亲电子试剂分别通过二电子和自由基途径在Ni(I)上独立活化。在该系统中,催化剂还原已被确定为限制周转的步骤。使用(Xantphos)Ni(I)Ar模型配合物仔细研究自由基形成步骤发现,Ni(I)通过一致的卤素吸收途径引发自由基形成.Ni的低氧化还原电势使我们能够开发还原性,反式选择性二烯环化反应,其中经典的两电子机制在Ni(I)/ Ni(III)平台上起作用,这说明了化学和立体选择性。该反应已发现在药物相关分子(例如3,4-二甲基加巴布丁)的有效合成中的应用。Ni经历单电子氧化还原过程的趋势促使我们探索双核Ni介导的键形成。这些研究提供了关于Ni-Ni键以及两个金属中心如何协同反应以促进CC,CX和NN键形成还原消除的见解。分离β-声镍和钯配合物可以对这些高反应性分子进行X射线和中子衍射表征。键合参数是β-声波相互作用的明确证据,有助于合理地消除相对于Pd的Ni处较慢的β-H消除。总的来说,我们的研究阐明了在多种情况下镍络合物的基本性质。更好的机械理解有助于催化剂设计,并有助于合理化Ni催化的烯烃官能化反应中的反应性和选择性。我们的研究阐明了在多种情况下镍络合物的基本性质。更好的机械理解有助于催化剂设计,并有助于合理化Ni催化的烯烃官能化反应的反应性和选择性。我们的研究阐明了在多种情况下镍络合物的基本性质。更好的机械理解有助于催化剂设计,并有助于合理化Ni催化的烯烃官能化反应中的反应性和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号