当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Advances in Rhodium-Catalyzed Oxidative Arene Alkenylation.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.accounts.0c00036 Weihao Zhu 1 , T Brent Gunnoe 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.accounts.0c00036 Weihao Zhu 1 , T Brent Gunnoe 1
Affiliation
|
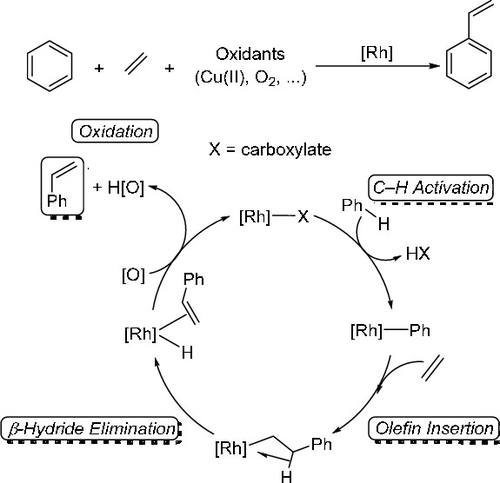
ConspectusAlkyl and alkenyl arenes are of substantial value in both large-scale and fine chemical processes. Billions of pounds of alkyl and alkenyl arenes are produced annually. Historically, the dominant method for synthesis of alkyl arenes is acid-catalyzed arene alkylation, and alkenyl arenes are often synthesized in a subsequent dehydrogenation step. But these methods have limitations that result from the catalytic mechanism including (1) common polyalkylation, which requires an energy intensive transalkylation process, (2) quantitative selectivity for Markovnikov products for arene alkylation using α-olefins, (3) for substituted arenes, regioselectivity that is dictated by the electronic character of the arene substituents, (4) inability to form alkenyl arenes in a single process, and (5) commonly observed slow reactivity with electron-deficient arenes. Transition-metal-catalyzed aryl-carbon coupling reactions can produce alkyl or alkenyl arenes from aryl halides. However, these reactions often generate halogenated waste and typically require a stoichiometric amount of metal-containing transmetalation reagent. Transition-metal-catalyzed arene alkylation or alkenylation that involves arene C-H activation and olefin insertion into metal-aryl bonds provides a potential alternative method to prepare alkyl or alkenylation arenes. Such reactions can circumvent carbocationic intermediates and, as a result, can overcome some of the limitations mentioned above. In particular, controlling the regioselectivity of the insertion of α-olefins into metal-aryl bonds provides a strategy to selectively synthesize anti-Markovnikov products. But, previously reported catalysts often show limited longevity and low selectivity for anti-Markovnikov products.In this Account, we present recent developments in single-step arene alkenylation using Rh catalyst precursors. The reactions are successful for unactivated hydrocarbons and exhibit unique selectivity. The catalytic production of alkenyl arenes operates via Rh-mediated aromatic C-H activation, which likely occurs by a concerted metalation-deprotonation mechanism, olefin insertion into a Rh-aryl bond, β-hydride elimination from the resulting Rh-hydrocarbon product, and net dissociation of alkenyl arene with formation of a Rh hydride. Reaction of the Rh hydride with Cu(II) oxidant completes the catalytic cycle. Although Rh nanoparticles can be formed under some conditions, mechanistic studies have revealed that soluble Rh species are likely responsible for the catalysis. These Rh catalyst precursors achieve high turnovers with >10,000 catalytic turnovers observed in some cases. Under anaerobic conditions, Cu(II) carboxylates are used as the oxidant. In some cases, aerobic recycling of Cu(II) oxidant has been demonstrated. Hence, the Rh arene alkenylation catalysis bears some similarities to Pd-catalyzed olefin oxidation (i.e., the Wacker-Hoechst process). The Rh-catalyzed arene alkenylation is compatible with some electron-deficient arenes, and they are selective for anti-Markovnikov products when using substituted olefins. Finally, when using monosubstituted arenes, consistent with a metal-mediated C-H activation process, Rh-catalyzed alkenylation of substituted arenes shows selectivity for meta- and para-alkenylation products.
中文翻译:

铑催化的氧化芳烃烯化反应的研究进展。
结论烷基和烯基芳烃在大规模和精细化学工艺中均具有重要价值。每年产生数十亿磅的烷基和烯基芳烃。历史上,合成烷基芳烃的主要方法是酸催化的芳烃烷基化,并且烯基芳烃通常在随后的脱氢步骤中合成。但是这些方法的局限性是由以下催化机理引起的:(1)普通的多烷基化,这需要能量密集的烷基转移过程;(2)马尔可夫尼可夫产物对使用α-烯烃进行芳烃烷基化的定量选择性;(3)对取代的芳烃,区域选择性这是由芳烃取代基的电子特性决定的;(4)无法在单个过程中形成烯基芳烃,(5)通常观察到与缺乏电子的芳烃反应缓慢。过渡金属催化的芳基碳偶联反应可从芳基卤化物产生烷基或烯基芳烃。然而,这些反应通常产生卤化废物,并且通常需要化学计量的含金属的重金属化试剂。涉及芳烃CH活化和烯烃插入金属-芳基键的过渡金属催化的芳烃烷基化或烯基化提供了制备烷基或烯基化芳烃的潜在替代方法。此类反应可避开碳阳离子中间体,因此可克服上述某些限制。特别地,控制α-烯烃插入金属-芳基键中的区域选择性提供了选择性合成抗马尔科夫尼科夫产物的策略。但是,以前报道的催化剂通常显示出抗马尔可夫尼可夫产品的寿命有限和选择性低的特点。因此,我们介绍了使用Rh催化剂前体进行单步芳烃烯基化反应的最新进展。该反应对于未活化的烃是成功的,并且表现出独特的选择性。链烯基芳烃的催化生产是通过Rh介导的芳族CH活化而进行的,这很可能是由于一致的金属化-去质子化机理,烯烃插入Rh-芳基键,从所得Rh-烃产物中除去β-氢化物和净解离烯基芳烃的合成,形成Rh氢化物。氢化铑与Cu(II)氧化剂的反应完成了催化循环。尽管在某些条件下可以形成Rh纳米颗粒,机理研究表明,可溶性Rh可能是催化作用的原因。这些Rh催化剂前体可实现高周转率,在某些情况下可观察到> 10,000的催化周转率。在厌氧条件下,使用羧酸铜(II)作为氧化剂。在某些情况下,已证明有氧循环利用Cu(II)氧化剂。因此,Rh芳烃的烯基化催化与Pd催化的烯烃氧化(即Wacker-Hoechst工艺)具有某些相似之处。Rh催化的芳烃烯基化与某些缺电子的芳烃相容,并且当使用取代的烯烃时,它们对反马尔科夫尼科夫产物具有选择性。最后,当使用单取代的芳烃时,与金属介导的CH活化过程一致,
更新日期:2020-04-23
中文翻译:

铑催化的氧化芳烃烯化反应的研究进展。
结论烷基和烯基芳烃在大规模和精细化学工艺中均具有重要价值。每年产生数十亿磅的烷基和烯基芳烃。历史上,合成烷基芳烃的主要方法是酸催化的芳烃烷基化,并且烯基芳烃通常在随后的脱氢步骤中合成。但是这些方法的局限性是由以下催化机理引起的:(1)普通的多烷基化,这需要能量密集的烷基转移过程;(2)马尔可夫尼可夫产物对使用α-烯烃进行芳烃烷基化的定量选择性;(3)对取代的芳烃,区域选择性这是由芳烃取代基的电子特性决定的;(4)无法在单个过程中形成烯基芳烃,(5)通常观察到与缺乏电子的芳烃反应缓慢。过渡金属催化的芳基碳偶联反应可从芳基卤化物产生烷基或烯基芳烃。然而,这些反应通常产生卤化废物,并且通常需要化学计量的含金属的重金属化试剂。涉及芳烃CH活化和烯烃插入金属-芳基键的过渡金属催化的芳烃烷基化或烯基化提供了制备烷基或烯基化芳烃的潜在替代方法。此类反应可避开碳阳离子中间体,因此可克服上述某些限制。特别地,控制α-烯烃插入金属-芳基键中的区域选择性提供了选择性合成抗马尔科夫尼科夫产物的策略。但是,以前报道的催化剂通常显示出抗马尔可夫尼可夫产品的寿命有限和选择性低的特点。因此,我们介绍了使用Rh催化剂前体进行单步芳烃烯基化反应的最新进展。该反应对于未活化的烃是成功的,并且表现出独特的选择性。链烯基芳烃的催化生产是通过Rh介导的芳族CH活化而进行的,这很可能是由于一致的金属化-去质子化机理,烯烃插入Rh-芳基键,从所得Rh-烃产物中除去β-氢化物和净解离烯基芳烃的合成,形成Rh氢化物。氢化铑与Cu(II)氧化剂的反应完成了催化循环。尽管在某些条件下可以形成Rh纳米颗粒,机理研究表明,可溶性Rh可能是催化作用的原因。这些Rh催化剂前体可实现高周转率,在某些情况下可观察到> 10,000的催化周转率。在厌氧条件下,使用羧酸铜(II)作为氧化剂。在某些情况下,已证明有氧循环利用Cu(II)氧化剂。因此,Rh芳烃的烯基化催化与Pd催化的烯烃氧化(即Wacker-Hoechst工艺)具有某些相似之处。Rh催化的芳烃烯基化与某些缺电子的芳烃相容,并且当使用取代的烯烃时,它们对反马尔科夫尼科夫产物具有选择性。最后,当使用单取代的芳烃时,与金属介导的CH活化过程一致,











































 京公网安备 11010802027423号
京公网安备 11010802027423号