当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Time-Resolved Photoelectron Spectroscopy Studies of Isoxazole and Oxazole.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-05-08 , DOI: 10.1021/acs.jpca.9b11788 Ting Geng 1 , Johannes Ehrmaier 2 , Oliver Schalk 1, 3 , Gareth W Richings 4 , Tony Hansson 1 , Graham Worth 5 , Richard D Thomas 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-05-08 , DOI: 10.1021/acs.jpca.9b11788 Ting Geng 1 , Johannes Ehrmaier 2 , Oliver Schalk 1, 3 , Gareth W Richings 4 , Tony Hansson 1 , Graham Worth 5 , Richard D Thomas 1
Affiliation
|
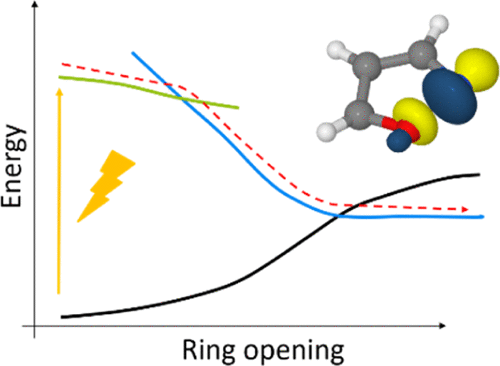
The excited state relaxation pathways of isoxazole and oxazole upon excitation with UV-light were investigated by nonadiabatic ab initio dynamics simulations and time-resolved photoelectron spectroscopy. Excitation of the bright ππ*-state of isoxazole predominantly leads to ring-opening dynamics. Both the initially excited ππ*-state and the dissociative πσ*-state offer a combined barrier-free reaction pathway, such that ring-opening, defined as a distance of more than 2 Å between two neighboring atoms, occurs within 45 fs. For oxazole, in contrast, the excited state dynamics is about twice as slow (85 fs) and the quantum yield for ring-opening is lower. This is caused by a small barrier between the ππ*-state and the πσ*-state along the reaction path, which suppresses direct ring-opening. Theoretical findings are consistent with the measured time-resolved photoelectron spectra, confirming the timescales and the quantum yields for the ring-opening channel. The results indicate that a combination of time-resolved photoelectron spectroscopy and excited state dynamics simulations can explain the dominant reaction pathways for this class of molecules. As a general rule, we suggest that the antibonding σ*-orbital located between the oxygen atom and a neighboring atom of a five-membered heterocyclic system provides a driving force for ring-opening reactions, which is modified by the presence and position of additional nitrogen atoms.
中文翻译:

异恶唑和恶唑的时间分辨光电子能谱研究。
通过非绝热从头算动力学模拟和时间分辨光电子能谱研究了异恶唑和恶唑在紫外光激发下的激发态弛豫途径。激发异恶唑的亮ππ*状态主要导致开环动力学。初始激发的ππ*状态和解离的πσ*状态都提供了无障碍的组合反应路径,因此开环定义为两个相邻原子之间的距离大于2Å,发生在45 fs内。相反,对于恶唑,激发态动力学大约慢两倍(85 fs),开环的量子产率更低。这是由于沿着反应路径的ππ*状态和πσ*状态之间的小势垒引起的,该势垒抑制了直接开环。理论发现与测得的时间分辨光电子能谱一致,证实了开环通道的时间尺度和量子产率。结果表明,时间分辨光电子能谱和激发态动力学模拟的结合可以解释此类分子的主要反应途径。作为一般规则,我们建议位于五元杂环系统的氧原子与相邻原子之间的反键σ*轨道为开环反应提供驱动力,该驱动力会因存在额外的氮原子。结果表明,时间分辨光电子能谱和激发态动力学模拟的结合可以解释此类分子的主要反应途径。作为一般规则,我们建议位于五元杂环系统的氧原子与相邻原子之间的反键σ*轨道为开环反应提供驱动力,该驱动力会因存在额外的氮原子。结果表明,时间分辨光电子能谱和激发态动力学模拟的结合可以解释此类分子的主要反应途径。作为一般规则,我们建议位于五元杂环系统的氧原子与相邻原子之间的反键σ*轨道为开环反应提供驱动力,该驱动力会因存在额外的氮原子。
更新日期:2020-04-03
中文翻译:

异恶唑和恶唑的时间分辨光电子能谱研究。
通过非绝热从头算动力学模拟和时间分辨光电子能谱研究了异恶唑和恶唑在紫外光激发下的激发态弛豫途径。激发异恶唑的亮ππ*状态主要导致开环动力学。初始激发的ππ*状态和解离的πσ*状态都提供了无障碍的组合反应路径,因此开环定义为两个相邻原子之间的距离大于2Å,发生在45 fs内。相反,对于恶唑,激发态动力学大约慢两倍(85 fs),开环的量子产率更低。这是由于沿着反应路径的ππ*状态和πσ*状态之间的小势垒引起的,该势垒抑制了直接开环。理论发现与测得的时间分辨光电子能谱一致,证实了开环通道的时间尺度和量子产率。结果表明,时间分辨光电子能谱和激发态动力学模拟的结合可以解释此类分子的主要反应途径。作为一般规则,我们建议位于五元杂环系统的氧原子与相邻原子之间的反键σ*轨道为开环反应提供驱动力,该驱动力会因存在额外的氮原子。结果表明,时间分辨光电子能谱和激发态动力学模拟的结合可以解释此类分子的主要反应途径。作为一般规则,我们建议位于五元杂环系统的氧原子与相邻原子之间的反键σ*轨道为开环反应提供驱动力,该驱动力会因存在额外的氮原子。结果表明,时间分辨光电子能谱和激发态动力学模拟的结合可以解释此类分子的主要反应途径。作为一般规则,我们建议位于五元杂环系统的氧原子与相邻原子之间的反键σ*轨道为开环反应提供驱动力,该驱动力会因存在额外的氮原子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号