当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Continuous Process Improvement in the Manufacture of Carfilzomib, Part 1: Process Understanding and Improvements in the Commercial Route to Prepare the Epoxyketone Warhead
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.oprd.0c00051 Peter K. Dornan 1 , Travis Anthoine 1 , Matthew G. Beaver 1 , Guilong Charles Cheng 1 , Dawn E. Cohen 1 , Sheng Cui 1 , William E. Lake 1 , Neil F. Langille 1 , Susan P. Lucas 1 , Jenil Patel 1 , William Powazinik 1 , Scott W. Roberts 2 , Chris Scardino 2 , John L. Tucker 2 , Simone Spada 1 , Alicia Zeng 1 , Shawn D. Walker 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.oprd.0c00051 Peter K. Dornan 1 , Travis Anthoine 1 , Matthew G. Beaver 1 , Guilong Charles Cheng 1 , Dawn E. Cohen 1 , Sheng Cui 1 , William E. Lake 1 , Neil F. Langille 1 , Susan P. Lucas 1 , Jenil Patel 1 , William Powazinik 1 , Scott W. Roberts 2 , Chris Scardino 2 , John L. Tucker 2 , Simone Spada 1 , Alicia Zeng 1 , Shawn D. Walker 1
Affiliation
|
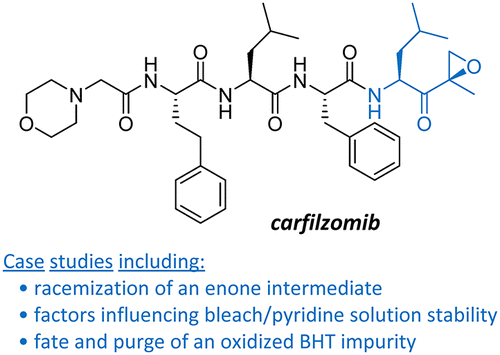
Epoxyketone 4 is an isolated intermediate in the manufacturing route to the commercial proteasome inhibitor carfilzomib (Kyprolis). Commercial process development and optimization efforts toward the preparation of epoxyketone 4 highlighted several opportunities for process improvement. In this article, three case studies are presented that demonstrate how a detailed understanding of the reaction mechanism led to improvements that increased the overall robustness of the process. In the first case study, the mechanism of racemization of an α-chiral enone was investigated, resulting in the development of an improved aqueous workup procedure. Next, the stability of a bleach/pyridine mixture used for the step 3 epoxidation reaction was studied, leading to the identification of pyridine as a key raw material and improved reaction conditions and control strategy to meet the conversion target. Finally, oxidized butylated hydroxytoluene (oBHT) was identified as an impurity arising from the use of BHT-stabilized tetrahydrofuran in steps preceding the oxidation. The process understanding obtained from these investigations led to the implementation of process improvements that improved the robustness of the process. The development of a second-generation route to 4 is the subject of part 2 in this series (DOI: 10.1021/acs.oprd.0c00052).
中文翻译:

卡非佐米生产中的连续过程改进,第1部分:制备环氧酮战斗部的工艺理解和商业路线的改进
环氧酮4是商业蛋白酶体抑制剂卡非佐米(Kyprolis)生产过程中的分离中间体。商业流程开发和优化工作,以制备环氧酮4强调了一些改进流程的机会。在本文中,提出了三个案例研究,这些案例研究证明了对反应机理的详细了解如何导致改进,从而提高了过程的整体稳定性。在第一个案例研究中,研究了α-手性烯酮外消旋化的机理,从而开发了改进的水后处理程序。接下来,研究了用于步骤3环氧化反应的漂白剂/吡啶混合物的稳定性,从而确定了吡啶作为关键原料,并改善了反应条件和控制策略,以达到转化目标。最后,氧化的丁基化羟基甲苯(oBHT)被确定为是在氧化之前的步骤中使用BHT稳定的四氢呋喃产生的杂质。从这些调查中获得的过程理解导致了过程改进的实施,从而改进了过程的鲁棒性。第二代路线的发展4是本系列第2部分的主题(DOI:10.1021 / acs.oprd.0c00052)。
更新日期:2020-04-24
中文翻译:

卡非佐米生产中的连续过程改进,第1部分:制备环氧酮战斗部的工艺理解和商业路线的改进
环氧酮4是商业蛋白酶体抑制剂卡非佐米(Kyprolis)生产过程中的分离中间体。商业流程开发和优化工作,以制备环氧酮4强调了一些改进流程的机会。在本文中,提出了三个案例研究,这些案例研究证明了对反应机理的详细了解如何导致改进,从而提高了过程的整体稳定性。在第一个案例研究中,研究了α-手性烯酮外消旋化的机理,从而开发了改进的水后处理程序。接下来,研究了用于步骤3环氧化反应的漂白剂/吡啶混合物的稳定性,从而确定了吡啶作为关键原料,并改善了反应条件和控制策略,以达到转化目标。最后,氧化的丁基化羟基甲苯(oBHT)被确定为是在氧化之前的步骤中使用BHT稳定的四氢呋喃产生的杂质。从这些调查中获得的过程理解导致了过程改进的实施,从而改进了过程的鲁棒性。第二代路线的发展4是本系列第2部分的主题(DOI:10.1021 / acs.oprd.0c00052)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号