当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Mizoroki–Heck Reaction of Nitroarenes and Styrene Derivatives
Organic Letters ( IF 4.9 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.orglett.0c00983 Toshimasa Okita 1 , Kitty K. Asahara 1 , Kei Muto 1 , Junichiro Yamaguchi 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.orglett.0c00983 Toshimasa Okita 1 , Kitty K. Asahara 1 , Kei Muto 1 , Junichiro Yamaguchi 1
Affiliation
|
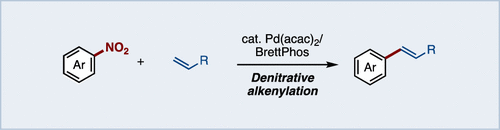
We have developed a Mizoroki–Heck reaction of nitroarenes with alkenes under palladium catalysis. The use of a Pd/BrettPhos catalyst promoted the alkenylation, whereas other catalysts led to a decrease in the product yield. In addition to nitroarenes, nitroheteroarenes were also applicable to the present reaction. The combination of a nucleophilic aromatic substitution (SNAr) with the denitrative alkenylation produced a multifunctionalized arene in a one-pot operation.
中文翻译:

钯催化的硝基芳烃和苯乙烯衍生物的咪唑罗基-赫克反应
我们已经开发了钯催化下硝基芳烃与烯烃的Mizoroki-Heck反应。Pd / BrettPhos催化剂的使用促进了烯基化,而其他催化剂导致产物收率下降。除硝基芳烃外,硝基杂芳烃也适用于本反应。亲核芳族取代(S的组合Ñ AR)与denitrative烯基化产生在单罐操作的多官能化的芳烃。
更新日期:2020-04-24
中文翻译:

钯催化的硝基芳烃和苯乙烯衍生物的咪唑罗基-赫克反应
我们已经开发了钯催化下硝基芳烃与烯烃的Mizoroki-Heck反应。Pd / BrettPhos催化剂的使用促进了烯基化,而其他催化剂导致产物收率下降。除硝基芳烃外,硝基杂芳烃也适用于本反应。亲核芳族取代(S的组合Ñ AR)与denitrative烯基化产生在单罐操作的多官能化的芳烃。











































 京公网安备 11010802027423号
京公网安备 11010802027423号