当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brønsted Acid Catalyzed Enantioselective Assembly of Spirochroman-3,3-oxindoles.
Organic Letters ( IF 4.9 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.orglett.0c00587 Alavala Gopi Krishna Reddy 1 , Pedireddi Niharika 1 , Su Zhou 1 , Shi-Kun Jia 1 , Taoda Shi 1 , Xinfang Xu 1 , Yu Qian 1 , Wenhao Hu 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.orglett.0c00587 Alavala Gopi Krishna Reddy 1 , Pedireddi Niharika 1 , Su Zhou 1 , Shi-Kun Jia 1 , Taoda Shi 1 , Xinfang Xu 1 , Yu Qian 1 , Wenhao Hu 1
Affiliation
|
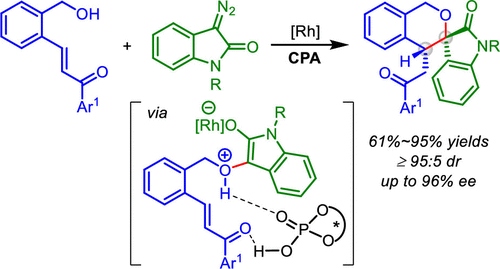
An enantioselective cyclization of diazoindolinones with o-hydroxymethyl chalcones has been established by a cooperative dirhodium complex and chiral phosphonic acid catalysis under mild conditions. This reaction is the first example of catalytic asymmetric intramolecular Michael-type trapping of oxonium ylide enabled by phosphoric acid through a dual H-bonding activation model, which provides an efficient access to the chiral spirochroman-3,3-oxindoles, with vicinal quaternary and tertiary stereocenters, in good to excellent yields and enantioselectivities.
中文翻译:

布朗斯台德酸对Spirochroman-3,3-oxindoles的对映选择性组装。
重氮配合配合物和手性膦酸催化在温和条件下建立了重氮吲哚酮与邻羟甲基查耳酮的对映选择性环化反应。该反应是通过双重H键活化模型实现磷酸催化的羟基叶立德催化不对称分子内迈克尔型Michael捕获的第一个例子,该模型可有效地连接手性spirochroman-3,3-oxindoles,以及邻位季铵盐和季铵盐。三级立体中心,具有良好的收率和对映选择性。
更新日期:2020-04-24
中文翻译:

布朗斯台德酸对Spirochroman-3,3-oxindoles的对映选择性组装。
重氮配合配合物和手性膦酸催化在温和条件下建立了重氮吲哚酮与邻羟甲基查耳酮的对映选择性环化反应。该反应是通过双重H键活化模型实现磷酸催化的羟基叶立德催化不对称分子内迈克尔型Michael捕获的第一个例子,该模型可有效地连接手性spirochroman-3,3-oxindoles,以及邻位季铵盐和季铵盐。三级立体中心,具有良好的收率和对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号