当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis and Antimicrobial Evaluation of 1,3,4‐Oxadiazole/1,2,4‐Triazole‐Substituted Thiophenes
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-01 , DOI: 10.1002/slct.202000191 Nishu Singla 1, 2, 3 , Gagandeep Singh 4 , Rohit Bhatia 2 , Anoop Kumar 2, 5 , Rupinder Kaur 2 , Satvinder Kaur 6
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-04-01 , DOI: 10.1002/slct.202000191 Nishu Singla 1, 2, 3 , Gagandeep Singh 4 , Rohit Bhatia 2 , Anoop Kumar 2, 5 , Rupinder Kaur 2 , Satvinder Kaur 6
Affiliation
|
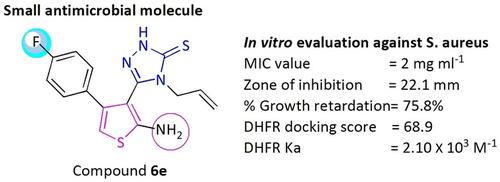
The increasing level of antimicrobial resistance in pathogenic bacteria, together with the lack of new potential drug scaffolds in the pipeline, make the problem of infectious diseases a major public health concern. Thus, in this context, a novel series of 1,3,4‐oxadiazole‐substituted thiophenes (4 a–m) and 1,2,4‐triazole (6 a–m) substituted thiophene derivatives were synthesized. Characterization of all the synthesized derivatives was done by various spectroscopic techniques such as 1H NMR, 13C NMR spectroscopy and mass spectrometry, and evaluated for antimicrobial activity against various pathological strains using broth dilution and disc diffusion method. In particular, compound 6 e and 4 e exhibited significant inhibitory potential with MIC ranging from 2–7 μg mL−1 against S. aureus, B. subtilis, P. aeruginosa and E. coli. Additionally, compound 6 e was found to be highly potent against methicillin resistant S. aureus (MRSA; MIC=2 μg mL−1). Molecular docking studies were also performed to confer the possible mode of action and association studies indicate the binding of potent active compound with DHFR enzyme (Ka=2.10×103 M−1). Further, the mechanism of action has also been explored by atomic force microscopy (AFM), which reveals the bacterial cell wall deformity and cell wall rupturing that may lead to bacteria cell death. Additionally, in silico ADME prediction study suggested the drug like properties of active compounds.
中文翻译:

1,3,4-恶二唑/ 1,2,4-三唑取代的噻吩的设计,合成和抗菌评估
致病细菌中抗菌素耐药性水平的提高,以及管道中缺乏新的潜在药物支架,使传染病成为主要的公共卫生问题。因此,在这种情况下,合成了一系列新的1,3,4-恶二唑取代的噻吩(4 a – m)和1,2,4-三唑(6 a – m)取代的噻吩衍生物。所有合成衍生物的表征均通过各种光谱技术进行,例如1 H NMR,13进行13 C NMR光谱和质谱分析,并使用肉汤稀释和圆盘扩散法评估其对各种病理菌株的抗菌活性。特别是,化合物6 È和4 ë表现出与MIC显著抑制潜力范围从2-7微克毫升-1针对金黄色葡萄球菌,枯草芽孢杆菌,铜绿假单胞菌和大肠杆菌。此外,化合物6 ë被发现是对耐甲氧西林高度有效的金黄色葡萄球菌(MRSA; MIC = 2微克毫升-1)。还进行了分子对接研究以赋予可能的作用方式,缔合研究表明有效的活性化合物与DHFR酶结合(K a = 2.10×10 3 M -1)。此外,还通过原子力显微镜(AFM)探索了作用机理,揭示了可能导致细菌细胞死亡的细菌细胞壁变形和细胞壁破裂。此外,计算机模拟ADME的预测研究表明活性化合物具有类似药物的特性。
更新日期:2020-04-01
中文翻译:

1,3,4-恶二唑/ 1,2,4-三唑取代的噻吩的设计,合成和抗菌评估
致病细菌中抗菌素耐药性水平的提高,以及管道中缺乏新的潜在药物支架,使传染病成为主要的公共卫生问题。因此,在这种情况下,合成了一系列新的1,3,4-恶二唑取代的噻吩(4 a – m)和1,2,4-三唑(6 a – m)取代的噻吩衍生物。所有合成衍生物的表征均通过各种光谱技术进行,例如1 H NMR,13进行13 C NMR光谱和质谱分析,并使用肉汤稀释和圆盘扩散法评估其对各种病理菌株的抗菌活性。特别是,化合物6 È和4 ë表现出与MIC显著抑制潜力范围从2-7微克毫升-1针对金黄色葡萄球菌,枯草芽孢杆菌,铜绿假单胞菌和大肠杆菌。此外,化合物6 ë被发现是对耐甲氧西林高度有效的金黄色葡萄球菌(MRSA; MIC = 2微克毫升-1)。还进行了分子对接研究以赋予可能的作用方式,缔合研究表明有效的活性化合物与DHFR酶结合(K a = 2.10×10 3 M -1)。此外,还通过原子力显微镜(AFM)探索了作用机理,揭示了可能导致细菌细胞死亡的细菌细胞壁变形和细胞壁破裂。此外,计算机模拟ADME的预测研究表明活性化合物具有类似药物的特性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号